Endotoxins can trigger severe inflammatory responses, which can result in conditions such as organ dysfunction, blood hypercoagulation, and systemic inflammatory response syndrome.
These risks make endotoxin testing essential in biomedical research and pharmaceutical manufacturing.
The Limulus Amebocyte Lysate (LAL) assay is currently the most common approach to endotoxin detection. It works by using blood cells from horseshoe crabs, which react to endotoxins through a specific aggregating response.
However, LAL assays are not completely specific and can produce false positives because they may also react with other substances. One such interfering substance is beta-glucan, a polysaccharide that can compromise the accuracy of endotoxin detection.
Despite its interference with LAL assays, β-glucan displays therapeutic potential when employed as an adjuvant in monoclonal antibody-based cancer immunotherapies. It enhances immune responses against tumors by stimulating complement receptor 3 (CR3).
In mouse models, antibodies conjugated with beta-glucan have achieved tumor inhibition rates of up to 86.7 % when combined with antibodies like anti-PD-L1.
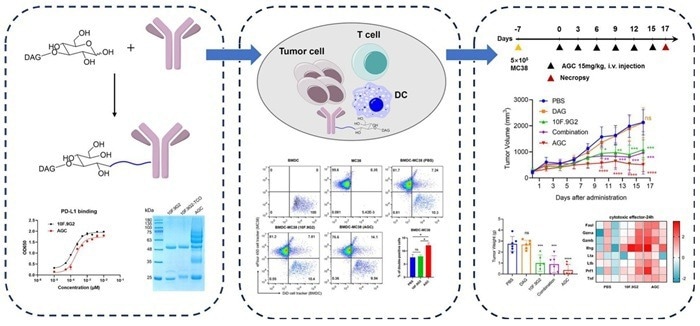
Anti-PD-L1 antibody coupled with β-glucan to construct AGC. Image Credit: ACROBiosystems
But β-glucan’s interference with LAL-based endotoxin testing complicates antibody drug development, however, because β-glucan’s tendency to cause false-positive results can lead to delayed drug approval and release.
Its widespread prevalence in algae, fungi, and synthetic fibers also makes β-glucan difficult to avoid in real-world applications.
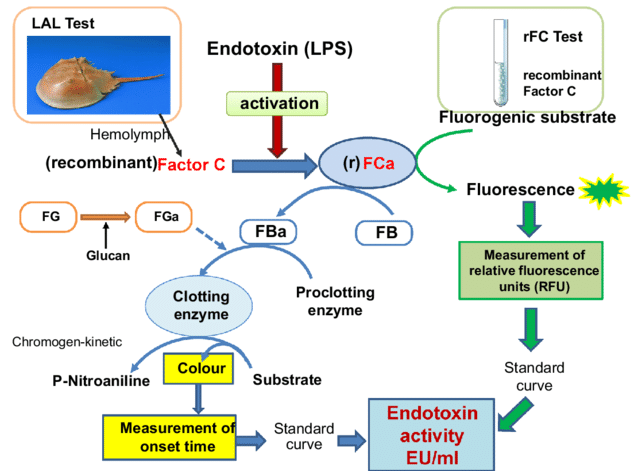
Schematic diagram of false positive endotoxin test due to β-glucan. Image Credit: ACROBiosystems
Overcoming false positives with recombinant Factor C assays
β-glucan-conjugated antibody (AGC) strategies offer significant promise for use in cancer immunotherapy. As a result, it’s essential to choose the appropriate endotoxin detection method during antibody drug development.
Research has shown that recombinant Factor C (rFC) assays offer major advantages in endotoxin detection compared to traditional LAL assays.
For example, testing for endotoxin contamination in β-glucan at concentrations of 10 μg/mL and 1 μg/mL revealed that the rFC assay showed no non-specific signals. This result verified its high specificity and capacity to avoid interference from β-glucan.
By contrast, testing with a dynamic chromogenic LAL assay produced non-specific signals in the presence of β-glucan, underscoring its vulnerability to interference.
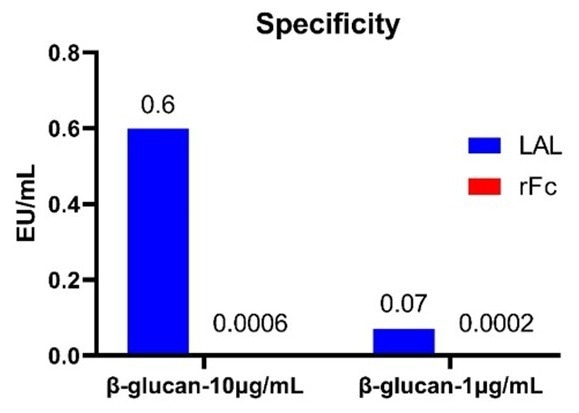
The recombinant Factor C assay for endotoxin is highly specific and unaffected by β-glucan. Image Credit: ACROBiosystems
Methods prone to β-glucan interference can produce inaccurate endotoxin readings when assessing antibody drugs, potentially compromising their quality and safety.
Because rFC assays are unaffected by β-glucan, these assays can be used to provide precise endotoxin measurements, providing drug developers with the reliable data necessary to ensure that antibody drugs meet all required quality standards.
By using rFC assays in drug development, success rate can be improved, supporting the development and manufacture of safe and effective antibody therapies.
Recombinant Factor C endotoxin detection kit
The recombinant Factor C endotoxin detection kit (Cat. No. RES-A056) from ACROBiosystems leverages the company’s proprietary platform for endpoint fluorescence measurement.
This kit uses powerful recombinant Factor C technology to provide precise, rapid endotoxin detection in samples, while ensuring that this detection remains free from interference by β-glucan.
The rFC endotoxin kit offers excellent sensitivity, specificity, and batch-to-batch consistency, making it ideally suited to meet the stringent quality control standards required in pharmaceutical manufacturing.
ACROBiosystems is actively advancing endotoxin detection technologies to support the development of safer, more effective therapies while reducing the impact on vital marine species, particularly horseshoe crabs.
Image Credit: ACROBiosystems
References and further reading
- Chan, G.C.-F., Chan, W.K. and Sze, D.M.-Y. (2009). The effects of β-glucan on human immune and cancer cells. Journal of Hematology & Oncology, (online) 2(1). https://doi.org/10.1186/1756-8722-2-25.
- Hong, F., et al. (2003). Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer research, (online) 63(24), pp.9023–31. Available at: https://pubmed.ncbi.nlm.nih.gov/14695221/.
- Wang, Q., et al. (2024). β-Glucan-conjugated anti-PD-L1 antibody enhances antitumor efficacy in preclinical mouse models. Carbohydrate polymers, (online) 324, p.121564. https://doi.org/10.1016/j.carbpol.2023.121564.
- DXY. (2022). Questions about G test and GM test for invasive fungal infection . (online) Available at: https://www.dxy.cn/bbs/newweb/pc/post/37303996 (Accessed 16 Jul. 2025).
- Liebers, V., Brüning, T. and Raulf, M. (2020). Occupational endotoxin exposure and health effects. Archives of Toxicology, 94(11), pp.3629–3644. https://doi.org/10.1007/s00204-020-02905-0.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.