Chimeric antigen receptor T, or CAR-T, cell therapy is a new therapy developed for a wide range of cancers. The main idea is to harvest T-cells from the patient and genetically engineer the cells in such a way that they express a chimeric receptor (CAR) which detects specific tumor-associated antigens (TAAs). Consequently, when the CAR-expressing T cells are reintroduced into the patient's body, they will target and remove the TAA-expressing tumor cells.
An important step in the production of CAR-T cells is the evaluation of CAR expression. This is usually achieved via flow cytometry, using protein L, anti-Fab antibodies, or target antigens as detection antibodies.
Among these common choices, target antigens are generally believed to be the best option as they provide minimal background staining and high specificity.
ACROBiosystems has produced a comprehensive collection of recombinant proteins to support the development of CAR-T therapy. This increasing list of proteins includes a number of pre-biotinylated proteins and fluorescent-labeled target antigens that are exclusively suitable for evaluating CAR expression. The company also supplies difficult-to-express proteins, such as EGFRVIII, ROR1, CD19, and BCMA.
CAR Detection Strategy and Product Design
Direct Method—Fluorescent-Labeled Proteins
Key Features
- Processing time can be decreased using direct-labeled proteins
- A fluorescent dye is used to pre-label target antigens
- Non-specific reaction of a secondary antibody is completely removed
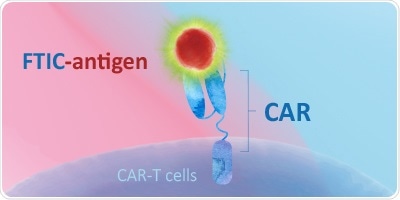
Image credit: ACRO Biosystems
FITC-Labeled -> Specially Designed
| BCMA |
CD19 |
CD22 |
GPC3 |
MSLN |
Protein L |
PE- Labeled -> Specially Designed
Case Study
Evaluation of Anti-CD19 CAR Expression with FITC-Labeled CD19
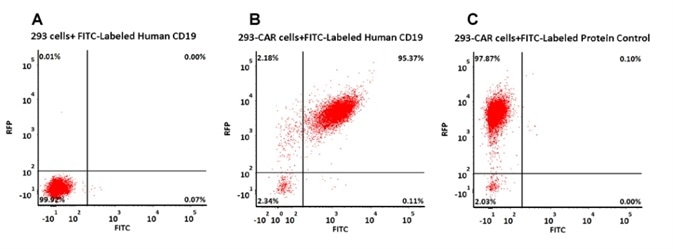
293 cells were transfected with anti-CD19-scFv and RFP tag. 2e5 of the cells were stained with B. FITC-Labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2, 10 µg/ml) and C. FITC-labeled protein control. A. Non-transfected 293 cells and C. FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2). Image credit: ACRO Biosystems
Biotin-Streptavidin Based Detection Using Biotinylated Proteins
Key Features
- Biotin is used to pre-label target antigens, which are detected by labeled streptavidin (the biotin-avidin complex)
- Streptavidin labeled with fluorochromes is capable of binding biotinylated proteins with a high degree of specificity and affinity, amplifying the signal, and enhancing the detection specificity and sensitivity
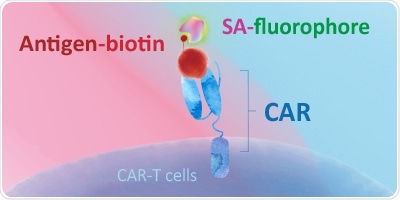
Image credit: ACRO Biosystems
Avitag TM Biotinylated Proteins -> Specially Designed
| BCMA |
CD22 |
CD30 |
CD33 |
CD38 |
CD70 |
| EGF R |
EGFRVIII |
EpCAM |
FOLR1 |
GPC3 |
HER2 |
| HGFR |
MSLN |
ROR1 |
|
|
|
Chemically Biotinylated Proteins -> Specially Designed
| CD19 |
EpCAM |
HER2 |
MSLN |
Protein L |
Case Study
Evaluation of Anti-BCMA CAR Expression with Biotinylated BCMA
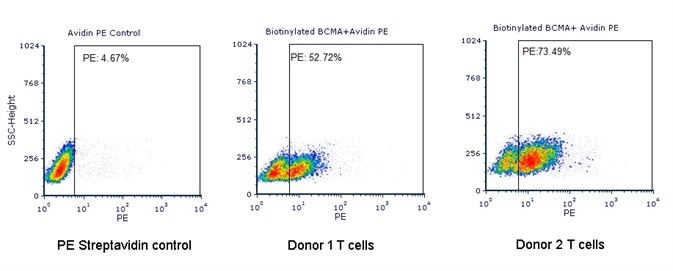
Human T cells were transfected with anti-BCMA CAR and cultured for three days. Three days post-transfection, 1e6 cells were first incubated with 50 µl biotinylated human BCMA protein (Cat. No. BC7-H82F0, 8 µg/ml), washed and then stained with PE Streptavidin and analyzed by flow cytometry. (Data are kindly provided by PREGENE Biopharma). Image credit: ACRO Biosystems
Indirect Detection Using Unconjugated Proteins
Key Features
- Target antigens are designed to perform a particular tag and are identified using a secondary antibody (anti-epitope tag antibody) labeled with a fluorophore
- Non-specific reaction of a secondary antibody may take place
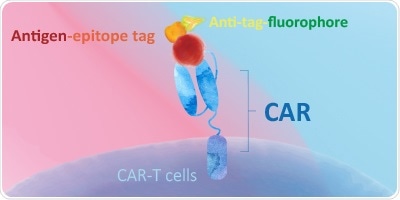
Image credit: ACRO Biosystems
Fc-Tag Fusion Proteins -> Specially Designed
| BCMA |
CD19 |
CD30 |
CD33 |
CD38 |
CD70 |
| EGFR |
FOLR1 |
GPC3 |
HER2 |
HGFR |
IL13RA2 |
| MSLN |
MUC1 |
ROR1 |
EpCAM |
|
|
His-Tag Fusion Proteins -> Specially Designed
| . |
. |
. |
. |
. |
. |
| BCMA |
CD19 |
CD123 |
CD138 |
CD22 |
CD30 |
| CD33 |
CD38 |
CD70 |
CAIX |
EGFR |
EGFRVIII |
| EpCAM |
FOLR1 |
GPC3 |
HER2 |
HGFR |
MSLN |
| ROR1 |
|
|
|
|
|
All Fusion Proteins -> Specially Designed
| . |
. |
. |
. |
. |
. |
| BCMA |
CD19 |
CD123 |
CD138 |
CD22 |
CD30 |
| CD33 |
CD38 |
CD70 |
CAIX |
EGFR |
EGFRVIII |
| EpCAM |
FOLR1 |
GPC3 |
HER2 |
HGFR |
IL13RA2 |
| MSLN |
MUCI |
ROR1 |
|
|
|
Case Study
Evaluation of Anti-CD19 CAR Expression with Fc-fusion CD19
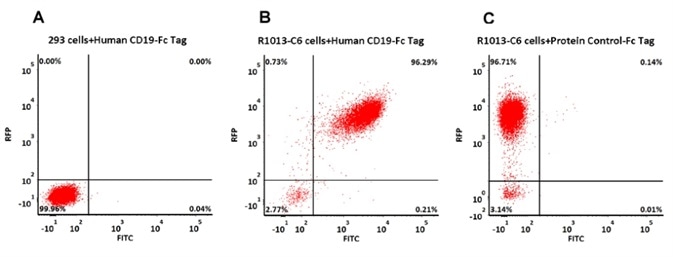
293 cells were transfected with FMC63-scFv and RFP tag. 2e5 of the cells were first stained with B. Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) (Cat. No. CD9-H5251, 3 µg/ml) and C. Human Fc Tag Protein Control, followed by FITC-conjugated Anti-human IgG Fc Antibody. A. Non-transfected 293 cells and C. Human Fc Tag Protein Control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) (Cat. No. CD9-H5251). Image credit: ACRO Biosystems
ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.