In therapeutic antibody development the fragment crystallizable (Fc) region is far more than just an antigen-binding aid; it's role is much larger, functioning as the antibody’s functional control center.
The Fc region governs four principal biological functions central to an antibody's therapeutic value via precise interactions with Fc gamma receptors (FcγRs, encompassing both activating and inhibitory subtypes) and the neonatal Fc receptor (FcRn). These are antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), complement-dependent cytotoxicity (CDC), and the regulation of antibody serum half-life.
From this core mechanism Fc engineering has evolved over time into the "engine" of therapeutic antibody development. It enables precise modulation of immune effector functions, optimization of pharmacokinetic (PK) characteristics, and even restructuring of target cell engagement patterns.
Ultimately, this approach improves therapeutic efficacy while reducing the likelihood of negative effects, creating new possibilities for addressing unmet clinical needs in complex diseases.
The following overview will systematically outline the three key directions of Fc engineering. It explores both the mechanisms of action for each approach and the integration of preclinical validation data and approved drug examples to connect technical principles with practical applications.
In addition, this article highlights key instruments that support Fc function analysis, equipping scientists with a complete reference framework from theoretical design to experimental validation.
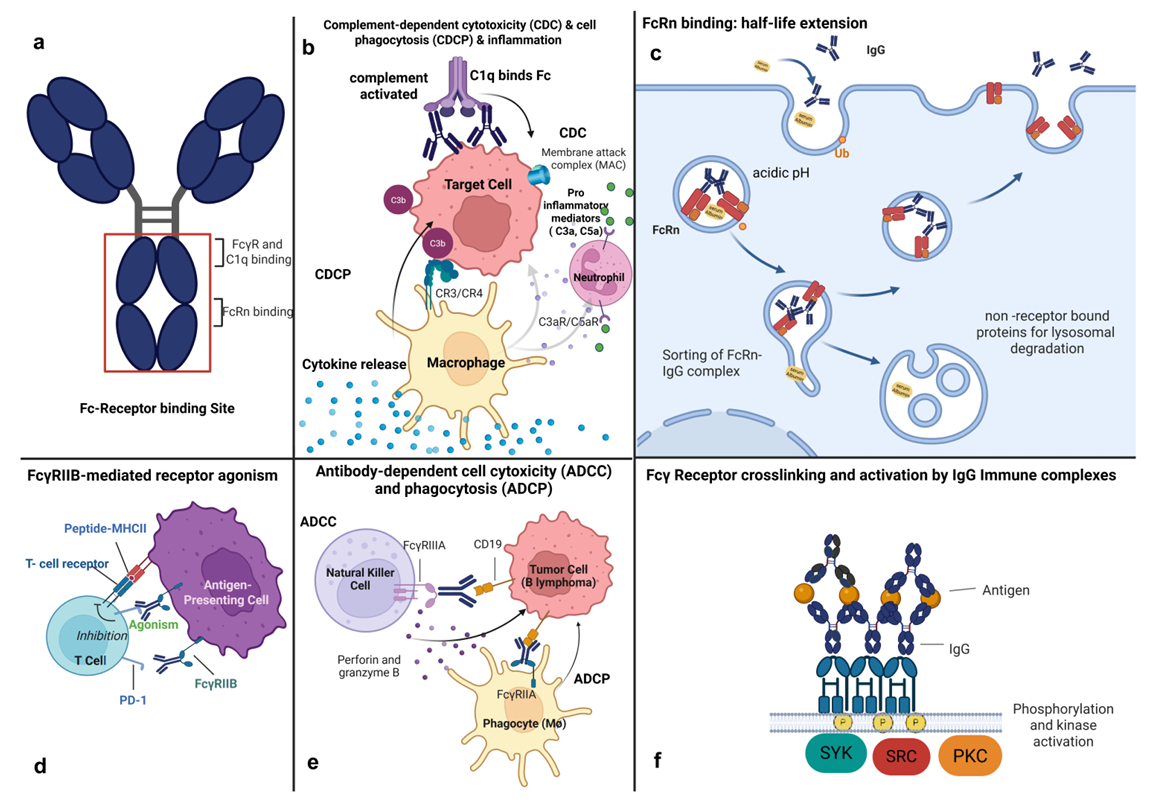
Figure 1. Safety-relevant aspects of enhanced Fc-effector function created in https://biorender.com (A) IgG structure indicating locations of critical immune receptor/ligand interactions. (B) Complement-related killing and immune cell activation. (C) IgG binding to FcRn in endothelial, macrophages, and other cells prolongs IgG half-life and hence exposure to its pharmacological activity, potentially increasing the risk of pharmacology-associated toxicity. (D). Receptor clustering driven directly by Ab format engineering or facilitated in trans by CD32b on a neighbouring cell can lead to receptor signalling and hence cell activation or inhibition, depending on receptor biology and cellular context. (E) Cellular killing mechanisms are effected through FcγRIIIa on NK cells (ADCC) or primarily FcγRIIa on phagocytic cells (ADCP). (F) IgG aggregates or target antigen-related immune complexes can activate FcγR, resulting in ‘off-target’ / ‘off biology’ related immune mediator/cytokine release toxicology. Image Credit: ACROBiosystems
Major directions of Fc engineering
The design behind Fc engineering always focuses on the therapeutic goals trying to be achieved. Where the elimination of tumor cells or pathogenic immune cells is required, Fc effector functions must be enhanced. When prevention of excessive immune activation is necessary, Fc effector functions must be silenced. Extending antibody half-life or directly blocking Fc receptor-mediated pathological processes involves two additional crucial optimization techniques.
While each of these four directions has distinct focuses, collectively they form the technical framework of Fc engineering.
I. Effector function enhancement or silencing: Precise regulation of immune clearance capacity
The "enhancement" and "silencing" of effector functions represent the most fundamental techniques in Fc engineering. They correspond to two diametrically opposed therapeutic scenarios: The need to activate immune clearance and the need to inhibit immune responses. Their technical pathways have been evaluated by several previous clinical studies.
1. Enhancing Fc effector functions: Activating the ‘killing potential’ of immune cells
Improving Fc-mediated effector activity is a fundamental technique in cancer treatment and certain autoimmune diseases requiring the clearance of pathogenic cells. At present, two technologies have emerged as gold standards in this sector, having been successfully translated into multiple approved therapeutics.
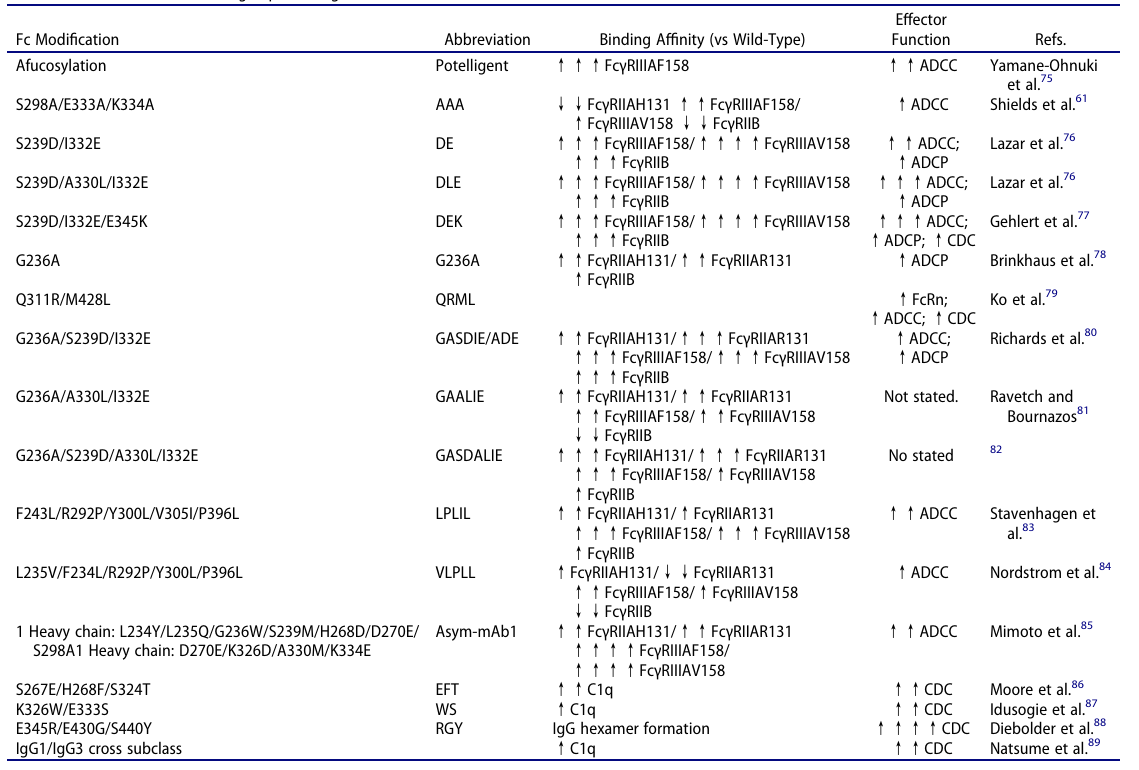
- Glycoengineering: Optimizing Fc Function through ‘Glycan Structure Modification’, a highly conserved N-glycosylation modification is located in the Asn297 site in the CH2 domain of the Fc region.
This seemingly small glycan chain is a key switch for Fc binding to FcγRs and C1q. In natural conditions, more than 90% of Fc glycans in human IgG contain core fucose. The modification of this glycan structure can directly alter Fc effector function:
Afucosylation: To date, the most clinically successful glycoengineering technique is afucosylation. Knocking out the FUT8 gene (responsible for fucose synthesis) in CHO cells or using naturally low-fucose producer cell lines can completely eliminate core fucose from Fc glycans.
This modification strengthens the binding affinity of Fc to the activating receptor FcγRIIIA (effective for both F158 and V158 allotypes), thereby improving NK cell-mediated ADCC activity. Clinically, this technology has become a standard feature in anti-CD20, anti-HER2, and other anti-tumor antibodies.
For example, afucosylation has been utilized to achieve therapeutic innovation in both Obinutuzumab (used in chronic lymphocytic leukemia) and Margetuximab (used in HER2-positive metastatic breast cancer).
Supplementary glycan modifications: Beyond afucosylation, optimized effector function can be achieved by adjusting other components of the glycan chain. For example, increasing galactose content in Fc glycans strengthens Fc binding to FcγRIIA/IIIA and C1q, thereby enhancing ADCP and CDC activity.
Introducing bisecting GlcNAc via overexpression of β1,4-N-acetylglucosaminyltransferase III (GnTIII) stabilizes Fc-FcγRIIIA interactions, leading to increased ADCC activity. Such modifications often serve as complementary methods to afucosylation, amplifying the killing effect of antibodies.
- Site-directed mutagenesis: Reshaping Fc conformation through amino acid modification
While glycoengineering focuses on "modifying the external glycan chains of Fc," site-directed mutagenesis focuses on optimizing the internal structure of Fc. Introducing specific amino acid mutations into Fc’s CH2/CH3 domains can directly modify the structural interface for Fc-receptor binding, thereby enabling accurate regulation of effector function.
Currently, multiple mutation combinations have passed preclinical validation, with several progressing to clinical stages:
-
- S239D/I332E (DE mutation): By modifying local Fc conformation, this set of mutations substantially strengthens binding to FcγRIIIA, ultimately boosting ADCC activity. It remains the most commonly used method for basic enhancement mutation.
- S239D/A330L/I332E (DLE mutation): Adding the A330L mutation to the DE backbone optimizes the Fc-FcγRIIIA binding interface, considerably increasing affinity for the FcγRIIIA V158 allotype and ADCC activity. This makes it well-suited for scenarios requiring increased effector function, such as solid tumor treatment.
- E345K/E430G mutation: This set of mutations functions through a distinct mechanism of action by promoting the formation of Fc hexamers after antibodies bind to target antigens.
- Hexamerized Fc recruits complement component C1q more efficiently, activating the classical complement pathway. In preclinical models, CDC activity can be enhanced, providing a novel optimization direction for antibodies dependent on complement-mediated killing, such as anti-CD20 antibodies.
- Q311R/M428E/N434W (REW mutation): This multifunctional mutation boosts CDC activity by promoting Fc hexamerization while optimizing FcRn binding sites to extend antibody half-life. As a result, it leads to both efficacy enhancement and PK optimization, providing new strategies for multidimensional antibody optimization.
Fc substitutions increasing FcγR binding. Source: ACROBiosystems
2. Silencing Fc effector functions: Avoiding ‘off-target damage’ from immune responses
Unlike cancer therapy, antibody treatment of autoimmune or inflammatory diseases involves blocking pathological signals instead of activating immune clearance. In this treatment, silencing Fc effector functions is essential to prevent antibodies from inadvertently activating immune cells or the complement system, minimizing damage to healthy tissues.
Key approaches for Fc silencing
- Targeted amino acid mutations
These mutations directly disrupt the structural interface between Fc and FcγRs/C1q, preventing undesirable effector activity while maintaining antigen binding. Three well-established mutation sets dominate clinical development:
-
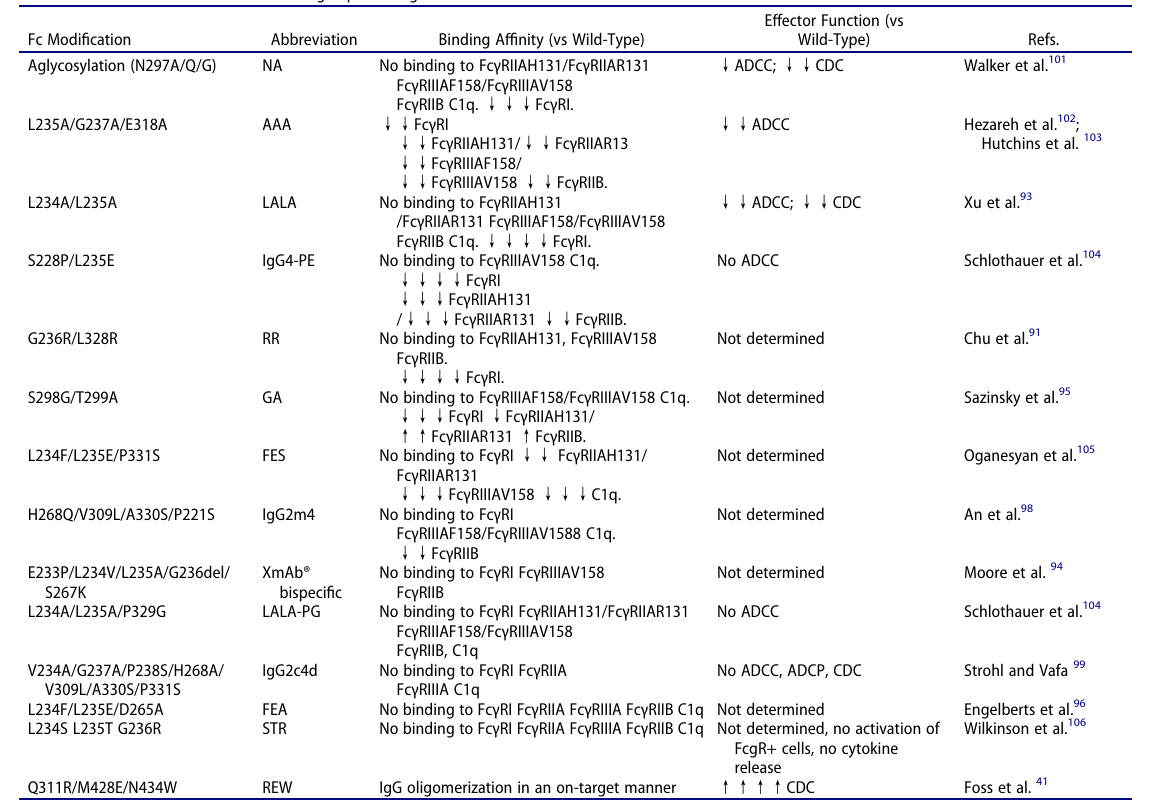
- N297A/Q/G: Blocks Fc glycosylation at Asn297, eliminating binding to low-affinity FcγRs (IIA/IIB/IIIA) and C1q, and reducing ADCC/CDC by more than 99% (may retain partial FcγRI binding).
- L234A/L235A (LALA): Weakens Fc binding to all FcγRs and C1q (100-1000× lower affinity) while preserving antigen specificity. This is commonly employed in IgG1 backbones, such as Atezolizumab, to prevent T cell depletion.
- L234A/L235A/P329G (LALA-PG): Builds on LALA to further reduce C1q binding, well-suited for complement-sensitive molecules such as bispecific antibodies like Glofitamab.
- IgG4 isotype optimization: Utilizing naturally low-activity isotypes to minimize risk
Alongside mutagenesis, choosing the naturally low-effector IgG4 isotype represents another important method for achieving Fc silencing. The isotype itself exhibits weak binding to FcγRs and C1q, but it carries a major drawback, easily undergoing Fab arm exchange in vivo, resulting in unstable antibody function.
To overcome this, the S228P mutation is usually introduced into the IgG4 hinge region to stabilize hinge disulfide bonds, completely blocking Fab arm exchange. For additional silencing, IgG4-S228P can be combined with the LALA mutation (e.g., the anti-IL-6R antibody sirukumab), effectively eliminating residual FcγR binding.
Critical consideration: Some silencing mutations, such as N297A, may impact antibody thermal stability or FcRm binding. Therefore, during the process of lead optimization, techniques like differential scanning calorimetry (DSC) and SPR should be used to evaluate these properties to maintain a balance between silencing efficacy, antibody stability, and half-life.
Fc amino acid substitutions ablating FcγR binding. Source: ACROBiosystems
II. Extending half-life via FcRn optimization: Improving the ‘clinical utility’ of antibodies
Dosing frequency and patient compliance are both impacted by an antibody’s serum half-life, which is regulated, though not determined, by the neonatal Fc receptor (FcRn). Working pH-dependently, FcRn binds IgG in acidic endosomes of pH 5.5–6.0 to prevent lysosomal degradation, which then releases IgG into neutral blood (pH 7.4).
Fc engineering targeting FcRn generally aims to extend half-life for improved convenience, though it can also adjust half-life for specific requirements.
- Extending half-life via FcRn optimization
Most efforts concentrate on strengthening acidic pH Fc-FcRn binding, with the effects of three validated mutation sets depending on factors such as antibody type and target:
-
- YTE (M252Y/S254T/T256E): Boosts hFcRn affinity by 6 to 11 times at pH 6.0; decreases clearance five to eight-fold in cynomolgus monkeys, and extends human half-life two to five-fold. May weaken ADCC (reversed by S239D/A330L/I332E mutations) (Dall’Acqua et al., 2006; Grevys et al., 2015).
- LS (M428L/N434S, Xtend™): Enhances hFcRn binding by 11.3 times at pH 6.0. Employed in Ravulizumab (anti-C5 for PNH), extending half-life to approximately 50 days (four times longer than eculizumab) (Lee et al., 2008; Peffault de Latour et al., 2018).
- N434H: Modestly improves FcRn affinity by approximately threefold, typically reducing clearance by about 50% in monkeys (Petkova et al., 2006; Valente et al., 2020). Assessed in anti-CD4 antibody MTRX1011A (autoimmune diseases) for potential dosage reduction, though clinical half-life is dependent on patient-specific factors.
Interestingly, these mutations may impair FcγR interactions and immune functions; YTE reduces ADCC activity, requiring a more balanced design. In addition, FcRn binding affinity varies across species, making human FcRn transgenic mice preferable for preclinical pharmacokinetic (PK) research to avoid inaccurate predictions.
III. Direct blockade of FcγRs and FcRn: Opening novel therapeutic pathways for receptor targeting
This approach uses Recombinant Fc fragments or multimers to competitively bind FcγRs/FcRn, preventing pathogenic IgG from interacting with these receptors. Primarily applied in the treatment of autoimmune diseases, this strategy overcomes the limitations of IVIG, such as limited supply, high cost, and long infusions, by providing targeted recombinant alternatives.
For FcγR blockade, multivalent Fc formats such as IgG1 Fc hexamers or IgG4 Fc trimers bind FcγRs to inhibit ADCC/ADCP. While IgG1 hexamers may induce platelet activation or cytokine release, IgG4-based hexamers minimize such risks and protect mice from autoimmune models, like ITP. Mice/NHPs lack human FcγRIIIB, thereby limiting data translatability.
For FcRn blockade, engineered Fc fragments such as efgartigimod, approved for gMG, contain mutations (e.g., MST-HN) that boost FcRn binding at acidic pH. This occupies FcRn, blocking the recycling of pathogenic IgG and accelerating its degradation. Clinical studies have demonstrated effective reductions in IgG and adequate safety.
Conclusion
Fc engineering is central to antibody drug development, allowing accurate modulation of immune functions and pharmacokinetics. Various therapeutic applications require customized Fc designs:
- Enhanced Fc for tumor cell clearance
- Silenced Fc for immune regulation and inflammatory control
- Extended half-life Fc for improved dosing convenience and patient compliance. Significant enhancements in therapeutic index and clinical outcomes can be achieved through advanced protein and glycan engineering methods.
Featured product line: High-performance Fc receptor tools - the ‘experimental cornerstone’ supporting Fc engineering
Reliable experimental tools are essential for the accurate implementation of the aforementioned Fc engineering approaches. The binding affinity, effector function, and PK properties of engineered Fc can only be accurately assessed using high-quality Fc receptor instruments. To satisfy this demand, a comprehensive portfolio of Fc receptor tools has been developed, including:
- Recombinant FcγR proteins
- TR-FRET binding assay kits
- Fc receptor functional cell lines
- Validated ADCC/ADCP reporter cell lines
To ensure data reliability and reproducibility, each product is validated using orthogonal techniques such as surface plasmon resonance (SPR), biolayer interferometry (BLI), and size exclusion chromatography-multi-angle light scattering (SEC-MALS).
Image Credit: ACROBiosystems
References and further reading:
- Brennan, F.R., et al. (2025). Impact of antibody Fc engineering on translational pharmacology, and safety: insights from industry case studies. mAbs, [online] 17(1). https://doi.org/10.1080/19420862.2025.2505092.
- Dall’Acqua, W.F., Kiener, P.A. and Wu, H. (2006). Properties of Human IgG1s Engineered for Enhanced Binding to the Neonatal Fc Receptor (FcRn). Journal of Biological Chemistry, 281(33), pp.23514–23524. https://doi.org/10.1074/jbc.m604292200.
- Grevys, A., et al. (2015). Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions. The Journal of Immunology, 194(11), pp.5497–5508. https://doi.org/10.4049/jimmunol.1401218.
- Lee, J.-W., et al. (2016). Immediate, Complete, and Sustained Inhibition of C5 with ALXN1210 Reduces Complement-Mediated Hemolysis in Patients with Paroxysmal Nocturnal Hemoglobinuria (PNH): Interim Analysis of a Dose-Escalation Study. Blood, 128(22), pp.2428–2428. https://doi.org/10.1182/blood.v128.22.2428.2428.
- Röth, A., et al. (2020). The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria. Blood, 135(12), 912–920. https://doi.org/10.1182/blood.2019003399
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.