World Myasthenia Gravis Day is celebrated every June 15th. Myasthenia gravis (MG) is an autoimmune illness that affects approximately 1.5 to 2 million people globally.
The disorder is characterized by fluctuating muscle weakness that often follows a "mild in the morning, worse in the evening" pattern. Patients experience fewer symptoms upon awakening and gradually deteriorate over the day.
Nearly 20 % of MG patients may experience life-threatening myasthenic crises, posing major risks to their health and survival.
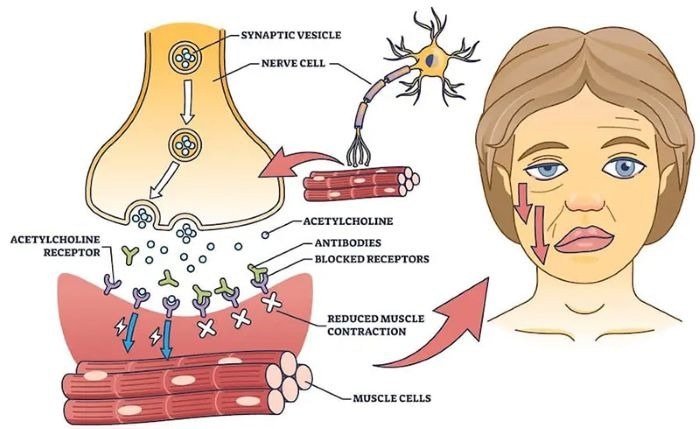
Pathogenesis and pathological features of MG. Image Credit: https://www.startstemcells.com/myasthenia-gravis.html
Corticosteroids are widely used in conventional therapy techniques. However, this medication has considerable side effects; long-term use can result in problems such as osteoporosis or metabolic syndrome, reducing patients' quality of life and limiting subsequent therapeutic efficacy.
While intravenous immunoglobulin (IVIG) therapy is effective, its high cost—more than $10,000 per infusion—makes it financially unaffordable for many patients, restricting its widespread adoption.
As a result, the development of safer, more cost-effective, and accessible targeted medicines is a critical need in the MG therapeutic landscape.

Image Credit: ACROBiosystems
MG: Neuromuscular signaling hijacking caused by misdirected immune responses
The basic pathology of MG is caused by an autoimmune attack on the neuromuscular junction (NMJ), in which the immune system incorrectly recognizes the acetylcholine receptor (AChR)—a crucial target at the NMJ—as a foreign antigen.
This leads to the development of pathogenic IgG autoantibodies. These antibodies inhibit neuromuscular signal transmission via several mechanisms:
- Primarily, the antibodies act as molecular plugs by specifically binding to AChRs, blocking acetylcholine (ACh) binding sites and directly impeding neurotransmitter-receptor interaction.
- Antibodies facilitate AChR internalization and degradation via cross-linking, reducing the availability of functional receptors at the NMJ.
- Antibody binding activates the complement cascade. Key components (C1q, C3, C5) undergo sequential activation, ultimately forming membrane attack complexes (MACs, C5b-9) at the NMJ. These molecular drills physically disrupt the postsynaptic membrane architecture, irreversibly destroying the foundation of signal transmission.
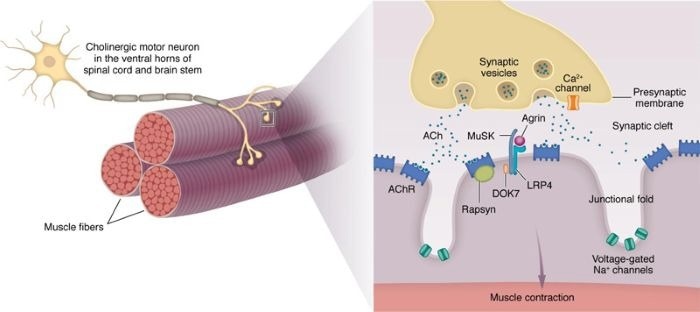
Structure of the NMJ. Image Credit: https://doi.org/10.1172/JCI179742
Simultaneously, the initiation and maintenance of the immune response in MG rely heavily on the driving functions of important immune cells and cytokines.
The basic immune attack mechanism is manifested as a cascade of events: pathogenic T helper 17 (Th17) cells act as major drivers, B cells/plasma cells produce antibodies, and the thymic microenvironment offers vital support.
Th17 cells that are specifically activated generate pro-inflammatory cytokines such as interleukin-17 (IL-17), IL-21, and IL-23, which drive B cell development into plasma cells (the primary source of pathogenic antibodies). Concurrently, regulatory T cell (Treg) malfunction impairs immunological tolerance.
IL-6 is a critical cytokine that stimulates the prolonged generation of autoantibodies by plasma cells. B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) promote the survival of B and plasma cells, while tumor necrosis factor-alpha (TNF-α) causes inflammation and impaired neuromuscular junction function.
About 10-15 % of patients have antibody subtype variants. For example, antibodies that target muscle-specific tyrosine kinase (MuSK) disrupt acetylcholine receptor (AChR) clustering.
Abnormal thymic tissue, which expresses self-antigens (e.g., AChR) and secretes IFN-γ and IL-1β, plays a crucial role in the autoimmune response, intensifying it.
The detailed understanding of these mechanisms is driving a shift in MG therapy methods away from non-specific immunosuppression and toward precision therapeutics that target specific pathways such as BAFF/APRIL and the neonatal Fc receptor (FcRn).
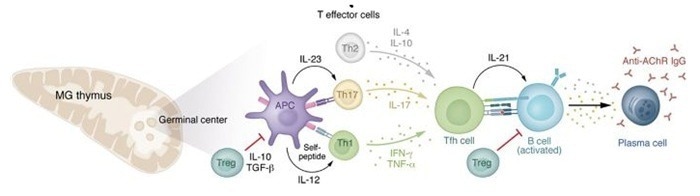
Cytokine network and immune cells in MG pathogenesis and immunoregulation. Image Credit: https://doi.org/10.1172/JCI179742
Breakthrough in treatment: From "symptom management" to precision targeting
MG treatment is shifting from "treating the symptoms without addressing the underlying disease" to precision targeting.
Conventional therapy has long depended on broad-spectrum suppression, with cholinesterase inhibitors (e.g., brompheniramine) in the symptomatic support stratum providing only temporary relief.
Glucocorticoids and classic immunosuppressive drugs (such as azathioprine and tacrolimus) in the immunosuppressive stratum are linked to serious infectious and metabolic side effects.
Plasma exchange (PLEX) and intravenous immunoglobulins (IVIg) in the antibody-clearing stratum provide instant relief, but they are temporary and expensive.
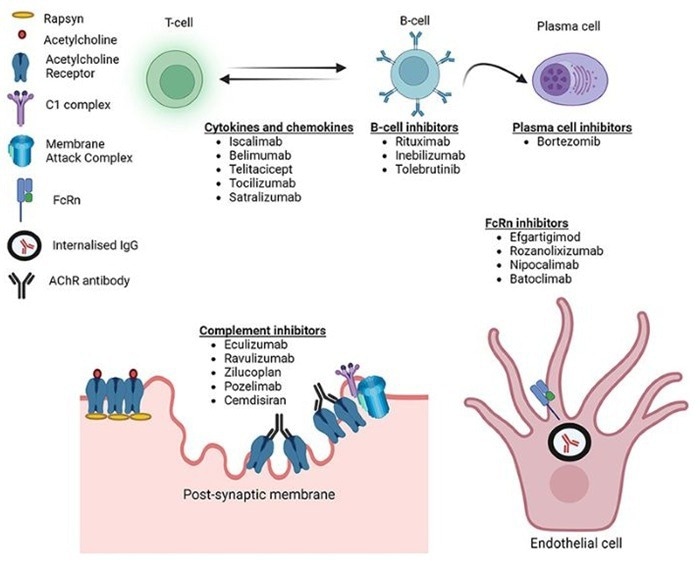
Targets of novel therapeutics in MG. Image Credit: https://doi.org/10.2147/ITT.S377056
The rise of targeted medicines marked a watershed moment, with market differentiation becoming increasingly important among licensed drugs. The FcRn antagonist section has experienced rapid expansion.
Efgartigimod has progressed into many indications, including MG and primary immune thrombocytopenia, with global revenues of $2.2 billion expected in 2024.
Its subcutaneous formulation, along with rozanolixizumab (sales of $227 million in 2024), is driving a revolution in dosing convenience. In April 2025, the FDA approved Johnson & Johnson's Nipocalimab, making it the first FcRn blocker to treat both anti-AChR/MuSK double-positive MG in adults and pediatric patients.
It may be injected subcutaneously in 30 seconds and is expected to generate $5 billion in sales at its peak. Among complement inhibitors, eculizumab had annual revenues of $210 million, while ravulizumab was approved in China for multiple sclerosis.
Traditional intravenous immunoglobulin (IVIg) saw a 12 % market decline in 2023 due to competition from innovative subcutaneous formulations.
Chinese novel medications are speeding market penetration: Telitacicept earned RMB 380 million in domestic sales in 2023, while Junshi Biosciences' toripalimab generated RMB 447 million in Q1 2025, representing a 45.7 % year-over-year rise and substantial market growth.
Pipeline development is centered on three transformational directions: dual-target/long-acting formulations, such as Povetacicept (a dual BAFF/APRIL antagonist), which obtained a 63 % full remission rate in Phase II studies for IgA nephropathy, with Phase III recruitment currently underway in China.
Gefurulimab (a C5/albumin bispecific antibody) increases the dose interval to eight weeks. Cell treatments are challenging traditional therapeutic paradigms.
Phase II data for Descartes-08 (an mRNA CAR-T treatment) in myasthenia gravis demonstrated that 100 % of patients improved significantly after 9 months, without the requirement for chemotherapeutic conditioning or the risk of cytokine release syndrome. The results of Phase 2b, expected in mid-2024, may alleviate safety obstacles.
Chinese pharmaceutical businesses are widely implementing distinct pipelines: Shanghai Pharmaceuticals' B007 (a humanized CD20 monoclonal antibody) has reached Phase II/III studies, needing only 5 minutes of subcutaneous administration—a 10-fold increase in dosage efficiency over Rituxima.
Tianchen Biotech's LP-005 is the world's first C3/C5 bispecific antibody that can suppress both complement activation nodes at the same time. Preclinical studies indicate a 40 % increase in blocking efficiency over single-target drugs.
Advancements in MG Drug Development. Source: Pharmacodia
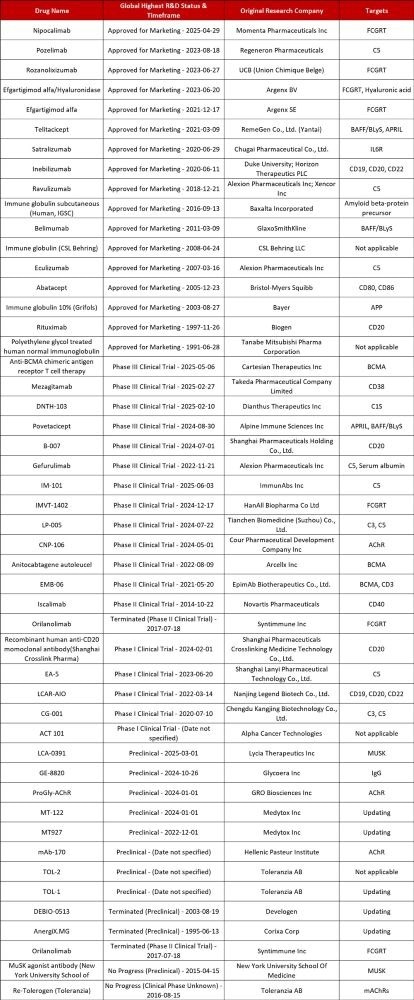
ACROBiosystems pioneers MG therapeutics with innovative solutions
The therapy of MG is changing dramatically from "indiscriminate suppression" to "precision-guided defusing."
With greater scientific understanding of the immune system's "faulty directives," as well as increased governmental backing and capital investment in the rare disease sector, the immunological shackles that bind individuals suffering from chronic weariness are being undone layer by layer.
ACROBiosystems offers a comprehensive product range for MG research, which includes highly active recombinant proteins, stable cell lines, and inhibitor screening kits.
ACROBiosystems’ solutions cover the entire drug development life cycle, from target discovery and validation to candidate drug screening and development, CMC manufacturing, and quality control, allowing for the efficient translation of MG innovative therapies from foundational research to clinical implementation.

Image Credit: ACROBiosystems
Hot MG target recommendations
Source: ACROBiosystems
| . |
. |
. |
. |
. |
| CD19 |
BCMA |
FcRn
(FCGRT & B2M) |
CD20 |
Complement C5 |
| 4-1BB |
APRIL |
CD40 |
Fc gamma
RIIB/C / CD32b/c |
BAFFR |
Fc gamma
RIIB / CD32b |
Complement C1s |
BAFF |
BCMA |
Complement C3 |
| CD38 |
IL-6 R alpha |
Serum Albumin |
... |
|
References
- Kaminski, H.J., et al. (2024). Myasthenia gravis: the future is here. The Journal of Clinical Investigation, (online) 134(12), p.e179742. https://doi.org/10.1172/JCI179742.
- Nair, S.S. and Jacob, S. (2023). Novel Immunotherapies for Myasthenia Gravis. ImmunoTargets and Therapy, Volume 12, pp.25–45. https://doi.org/10.2147/itt.s377056.
- Huda, R. (2023). Inflammation and autoimmune myasthenia gravis. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1110499.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.