In February 2025 Harbour BioMed announced that the IND application for HBM9378 (SKB378), a TSLP-targeting monoclonal antibody developed in collaboration with Kelun-Biotech Medicine for Chronic Obstructive Pulmonary Disease (COPD), had been approved by the National Medical Products Administration (NMPA) in China.
This approval officially marked the entry of their innovative antibody into clinical development, providing a potential new treatment for COPD patients.

Image Credit: Harbour BioMed Official Website News Center (https://www.harbourbiomed.com/news/232.html)
HBM9378 is the second fully human monoclonal antibody developed to target thymic stromal lymphopoietin (TSLP). It has been co-developed by Harbour BioMed and Kelun-Biotech Medicine, with rights held in Greater China and selected Southeast Asian and Middle Eastern markets.
Using Harbour BioMed's proprietary H2L2 Harbour Mice® platform, this antibody works by blocking the TSLP signaling pathway, which plays a key role in immune diseases such as asthma and COPD.
HBM9378 has optimized immunogenicity, bioavailability, and a half-life two to three times longer than some existing technologies. These properties substantially improve HBM9378’s dosing convenience.
On January 10, a global licensing agreement worth up to $1 billion was signed with Windward Bio AG. This agreement grants Windward exclusive rights to develop and commercialize HBM9378 outside of Greater China.
Phase I clinical trials in China for the treatment of moderate to severe asthma are complete and the drug is due to progress to Phase II.
TSLP: The next immune frontier surrounded by MNC
TSLP is an IL-2 class cytokine similar to IL-7, which plays a key role in immune responses, particularly in type 2 immunity.
Mechanistically, TSLP regulates immune responses by stimulating type 2 inflammatory cells to produce pro-inflammatory cytokines like IL-4, IL-5, and IL-13. Elevated TSLP levels can trigger immune diseases such as asthma, atopic dermatitis, and COPD.
Blocking TSLP may prevent these diseases from being triggered in patients, making it an ideal therapeutic target.
The only TSLP monoclonal antibody currently on the market is Amgen/AztraZeneca’s Tezepelumab, which received FDA approval in December 2021 for treating severe asthma in patients aged over 12-years-old.
However, the drug was only recently submitted for approval in China in November 2024. Beyond asthma, Tezepelumab is in ongoing clinical studies for multiple other diseases, including chronic sinusitis with nasal polyps, atopic dermatitis, and COPD.
Tezepelumab has experienced substantial growth in sales, with global revenue rising from $170 million in 2022 to $570 million in 2023, and reaching $840 million in the first nine months of 2024.
The successful launch of Tezepelumab has validated both the druggability and commercial promise of the TSLP target, drawing interest from several multinational pharmaceutical companies. Currently, around 27 TSLP-related drug candidates are in development worldwide, with major players such as Sanofi, GSK, AstraZeneca, and Pfizer actively involved. Among these, Sanofi’s IL-13/TSLP bispecific antibody, Lunsekimig, stands out for its rapid progress—now in phase 3 clinical trials for asthma and chronic sinusitis with nasal polyps.
Chinese biotech firms have taken a leading role in TSLP-related deals in recent years. Companies like Hengrui Medicine and Qsine Biosciences are expanding the scope of TSLP drug development through equity-based partnerships and NewCo structures, helping diversify the landscape of global collaborations.
Business Development Transactions of the TSLP Drug Pipelines. Source: ACROBiosystems
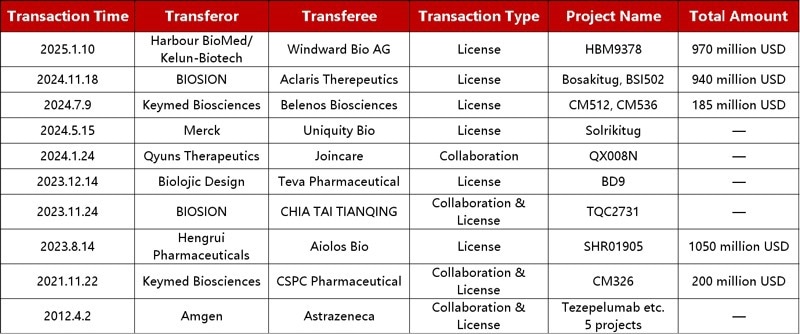
It is anticipated that the asthma therapy market will reach $9.7 billion in China by 2030, and the atopic dermatitis market will grow to $4.3 billion, driving domestic companies to accelerate their TSLP pipeline initiatives even more.
BIOSIN’s BSI-045B has demonstrated excellent performance in a Phase IIa single-arm proof-of-concept trial in the U.S., indicating its potential “first-in-class” value. Such was its performance that the stock price of Bio-Design’s partner Aclaris Therapeutics rose by 53 % on the same day as BSI-045B’s results were announced.
As the TSLP target pipeline gains momentum, companies focusing on such innovative approaches in this field are expected to become industry leaders.
High-quality TSLP tools support drug development for allergic and inflammatory diseases
ACROBiosystems has launched a series of high-quality biopharmaceutical development tools covering TSLP, TSLPR, IL-7 R alpha, and TSLP R.
These tools address drug development needs for allergic and inflammatory diseases, including asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, and COPD.
- TSLP recombinant proteins and TSLP R recombinant proteins: These proteins are expressed in human-derived HEK293 cells and validated by SEC-MALS, ELISA, SPR, and BLI, exhibiting high purity, activity, and batch-to-batch consistency. They are suitable for immunology applications, antibody screening, and validating candidate drug functionality.
- IL-7 R alpha & TSLP R heterodimer recombinant protein: This high-purity, high-activity protein with a dimeric structure is verified by SEC-MALS. It simulates the natural TSLPR complex binding with TSLP to initiate downstream signaling pathways, facilitating studies on TSLP-mediated signaling pathways and biological activity in vitro.
- TSLP [Biotinylated]: IL-7 R alpha & TSLP R inhibitor screening ELISA kit: This competitive ELISA method is designed to establish an inhibitor screening platform. Developed using unique biotinylated proteins, this high-quality kit has been tested for bispecific antibody drug screening. It is suitable for early-stage screening and QC quality control.
- Various high-quality targeted drug development tools: ACROBiosystems provides additional tools for allergy and inflammatory diseases, including IL-4, IL-5, IL-5 Rα, IL-13, and IgE Fc, fully supporting drug development research.
ACROBiosystems conducts batch-by-batch quality control on all products, verifying properties such as purity and binding activity, and offers free protocols to streamline integrin-targeted drug development.

Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.