Breast cancer is the most common type of cancer in women and is a considerable threat to public health.
Breast cancer is a heterogeneous disease that can be categorized into three significant clinically appropriate subtypes, which have been regulated differently: human epidermal growth factor receptor-2+ (HER-2+), luminal [expressing the progesterone receptor (PR), and estrogen receptor (ER)].
Triple-negative breast cancer (TNBC) is known to be the fiercest subtype of breast cancer and has been characterized by the lack of progesterone receptor (PR) expression, estrogen receptor (ER), and the lack of human epidermal growth factor receptor 2 (HER2) expression or amplification.
Traditional chemotherapy is considered to be the backbone of systemic treatment for TNBC. But the absence of targeted molecular therapies and the poor prognosis of TNBC patients have prompted a great need to discover effective targets for enhancing clinical outcomes.
There are three types of treatments for breast cancer that are currently available: 1) monoclonal antibodies that target tumor antigens; 2) immune checkpoint inhibitors like PD-1 and PD-L1 antibodies; 3) antibody-drug conjugates (ADC).
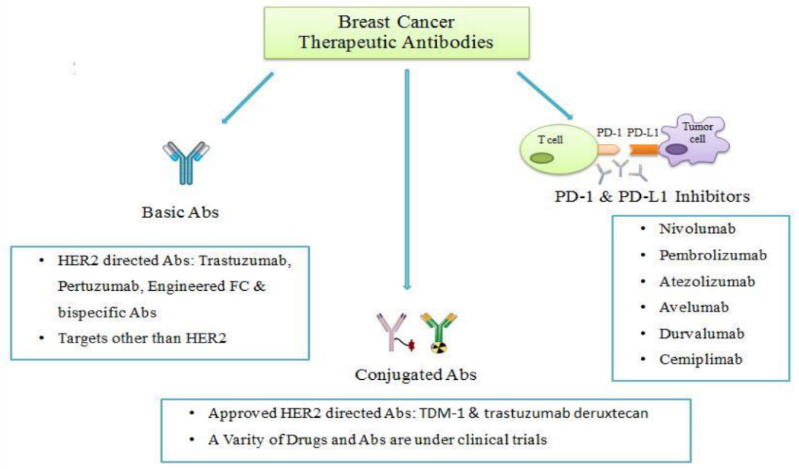
Figure 1. The main types of antibodies for the treatment of breast cancer (data from literature). Image Credit: ACROBiosystems
Monoclonal antibodies
By attaching to HER2 or other antigens on the tumor surface, monoclonal antibodies block the signaling pathways associated with tumor cell growth, inhibit tumor growth, or kill tumor cells through antibody-mediated ADCC and other mechanisms.
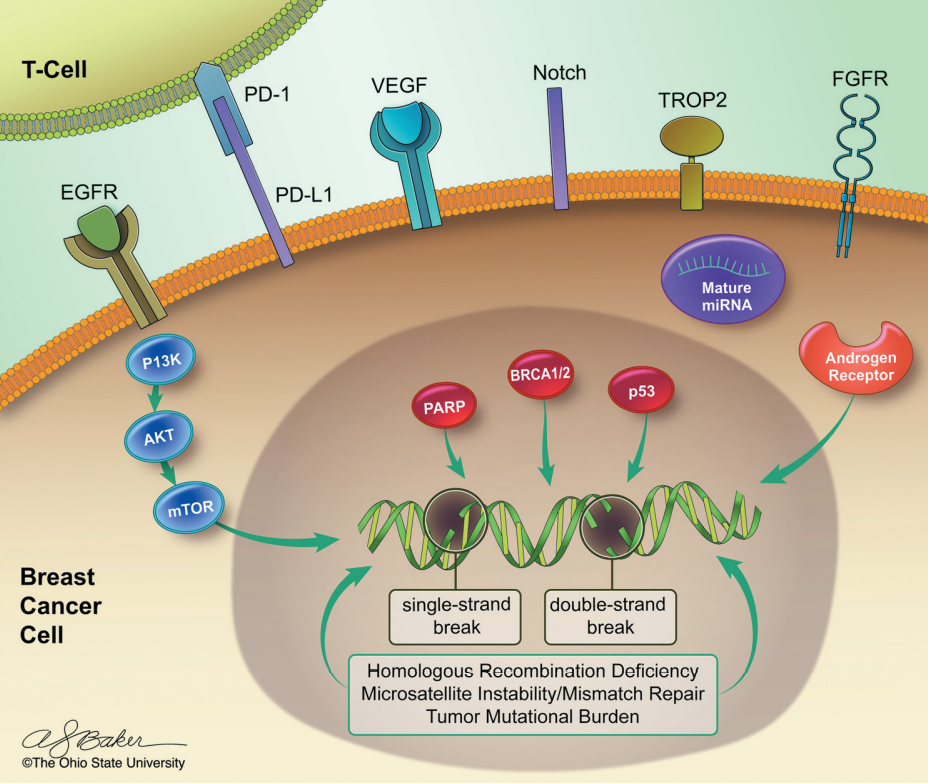
Figure 2. The key mechanism of signal transduction and tumorigenesis in triple-negative breast cancer (data from literature). Image Credit: ACROBiosystems
EGFR
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that comes under the ErbB family and is also involved in cell proliferation, angiogenesis, inhibition of apoptosis, and metastasis.
The EGFR protein is overexpressed more often in TNBC, according to studies, and is also an independent prognostic indicator of overall survival (OS) and disease-free survival (DFS).
In Phase II studies, the monoclonal antibody cetuximab coupled with cisplatin chemotherapy showed promising results, implying that some subtypes of TNBC may be sensitive to EGFR inhibition.
VEGF
Vascular Endothelial Growth Factor (VEGF) has been expressed by 30–60% in TNBC. It tends to stimulate endothelial cell proliferation and migration, hinders endothelial cell apoptosis, and encourages angiogenesis.
TNBC patients had significantly higher VEGF expression than non-TNBC patients, according to one study, and TNBC patients had shortened disease-free survival (DFS) than non-TNBC patients. Angiogenesis is thought to be a crucial factor in tumor cell proliferation.
As a result, VEGF has emerged as a promising therapeutic target for TNBC. According to the findings of the research on adjunctive bevacizumab (a monoclonal antibody that targets VEGF-A) in TNBC patients, bevacizumab may enhance the effectiveness of immunotherapy.
FGFR
Fibroblast growth factor receptors (FGFRs) are a type of transmembrane receptor that is involved in a variety of processes such as proliferation, migration, and angiogenesis. FGFR, its various FGF ligands, and signaling partners are frequently dysregulated in the progression of breast cancer and are one of the reasons for treatment resistance in breast cancer.
Furthermore, genetic changes in FGFRs may play a role in tumor progression and poor outcomes in breast cancer patients. As a result, FGFR is being looked at as a possible therapeutic target for breast cancer.
Several studies have found that abnormal activation of the FGF/FGFR axis plays a role in the progression of various breast cancer subtypes. Numerous subgroups of breast cancer patients have been found to have FGFR genetic abnormalities, including amplification of FGFR1, FGFR2, and FGFR4 and mutations in FGFR2 and FGFR4.
Even though FGFR1 amplification is linked to a weaker prognosis in hormone receptor-positive breast cancer, its role in TNBC is unclear. FGFR2 expression was linked to a lower overall survival rate. In recent years, FGFR inhibitors have become one of the most popular small molecule drugs. If patients with triple-negative breast cancer can also benefit from FGFR inhibitors, their chances of survival will be vastly enhanced.
The biggest roadblock to clinical approval is breast cancer patients’ resistance to FGFR inhibitor therapy. Preclinical data on the effectiveness of antibodies against FGFR isoforms and fibroblast growth factor ligand inhibitors in solid tumors, including BC, has shown promising results in phase I clinical trials.
NOTCH signaling pathway-related targets
NOTCH signaling triggers various genes linked to cell differentiation, proliferation, and death. Four heterodimeric transmembrane Notch receptors (1–4) react with the transmembrane ligands Delta-like (Dll) 1, 2, 4, and Jagged (JAG) 1 and 2 are determined in humans.
Studies have indicated that the Notch pathway has significant participation in breast cancer progression via overexpression and abnormal genetic type expression of the notch receptors and ligands that identify angiogenesis.
As far as breast cancer is concerned, functionally mutated NOTCH comprises around 10% of TNBC. Studies have displayed a correlation between NOTCH-1 and positive lymph node status and Jagged-1 and larger tumor status.
Also, studies have indicated that increased expression of NOTCH-1, NOTCH-4, or Jagged-1 is linked to detrimental prognostic factors like reduced survival.
Hence, targeting the inhibition of the NOTCH signaling pathway will be useful for breast cancer treatment. Numerous inhibitors have been created for this pathway development.
PRLR
The Prolactin Receptor (PRLR) is a type 1 cytokine receptor that has been expressed in a subset of breast cancers and could contribute to its pathogenesis. The Jak/Stat, PI3-kinase/AKT, and MAPK signaling pathways are triggered when PRLR is integrated with PRL, GH, or PL, thereby leading to tumor cell proliferation.
PRLR has been overexpressed in malignant breast epithelium compared to normal breast tissue and expressed throughout molecular subtypes of human breast cancers. The neutralizing antibody LFA-102 antagonizes PRLR signaling and proliferation in breast cancer cells and lapses PRL-dependent tumor xenografts.
There was a restriction in efficacy even though LFA-102 was tolerated as an unconjugated antibody in Phase 1 clinical trials in patients with prostate cancer and metastatic breast cancer.
Cop1
In spite of the unusual clinical efficacy of immune checkpoint blockade (ICB) in cancer treatment, ICB advantages for triple-negative breast cancer (TNBC) remain restricted. The immunosuppressive tumor microenvironment of TNBC decreases the effective response of tumors to those drugs.
Hence, if new targets are discovered to reprogram the tumor microenvironment, it will help enhance the efficacy of immunotherapy in TNBC.
A study reported in Cells suggested that deletion of the E3 ubiquitin ligase Cop1 in cancer cells reduces the secretion of macrophage-associated chemokines, decreases tumor macrophage infiltration, improves anti-tumor immunity, and reinforces ICB response. Thus, Cop1 is considered a target for enhancing cancer immunotherapy efficacy in TNBC.
In most cases, the immune system performs a crucial role in tumor prevention. Immune escape via a variety of mechanisms is a vital factor in the emergence and progression of malignant tumors. Breast cancer treatment has entered a new era thanks to immunotherapy.
As widely known, the PD-1 ligand PDL1 engages with T-cells leading to upregulation of the receptor pursued by an immunosuppressive signal. This prevents kinases that are involved in the activation of the immune response.
Pro-inflammatory cytokines and the overexpression of PDL1 inhibitory ligand could play a primary role in developing cancer immune resistance mechanisms, leading to a state of exhausted or tolerant immune T-cell response and hence the significance of studying the possible role of PDL1 expression as a resistance biomarker.
In addition, high PD-1 and PD-L1expression, both negative regulators of the local immune response, are linked to improved overall survival and a higher pCR rate in TNBC. Data from various clinical trials has approved the potential role of an immune checkpoint inhibitor in early-stage TNBC.
In 2019, the FDA approved Atezolizumab, a PD-L1 inhibitor, along with nab-paclitaxel (Abraxane) for the first-line therapy of metastatic TNBC, which is known to be the first immunotherapeutic for breast cancer.
The KEYNOTE-522 trial (NCT03036488) assessed the safety and efficacy of pembrolizumab plus chemotherapy as neoadjuvant therapy and came to the conclusion that pembrolizumab has good anti-tumor efficacy and controllable safety in first-line treatment of metastatic TNBC. Also, chemotherapy integrated with PD-1 monoclonal antibody can enhance the anti-tumor efficacy.
The KEYNOTE-355 phase III trial assessed the efficacy of pembrolizumab with chemotherapy compared to several chemotherapy regimens alone as first-line therapy for incurable TNBC (NCT02819518).
The outcomes presented at ESMO 2021 displayed that pembrolizumab integrated with chemotherapy considerably enhanced PFS and OS in PD-L1-positive patients.
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are a new class of extremely potent molecules that integrate the targeting properties of monoclonal antibodies with the cell-destroying properties of cytotoxic agents to minimize systemic exposure and toxicity of the latter.
ADCs have opened the door to a new era in breast cancer treatment. TROP-2, GPNMB (Osteoactivin), LIV-1, Cadherin-6, and other promising targets have been discovered in TNBC.
TROP-2
TROP-2 is a type I transmembrane glycoprotein. TROP-2 plays a significant role in cell migration, cell cycle progression and metastasis, and high expression in lung cancer, breast cancer, etc., thereby making it a huge target in tumor therapy.
On April 7th, 2021, Gilead declared the FDA approval of sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for unresectable locally advanced or metastatic triple-negative cancer patients breast cancer (mTNBC) who have got two or more prior systemic therapies, at least one of them taken for metastatic disease.
Trodelvy is an antibody-drug conjugate (ADC) made of a humanized RS7 antibody targeting Trop-2 linked with SN38 at a DAR of 7.6:1.
GPNMB
GPNMB is included in cell migration, angiogenesis, invasion, or epithelial-mesenchymal transition, highly overexpressed in TNBC. GPNMB is a poor prognosis biomarker in BC.
LIV-1
LIV-1 comes under the multi-span transmembrane protein of the solute carrier family 39 with putative zinc transporter and metalloproteinase activity. As a downstream target of STAT3, it encourages the epithelial-to-mesenchymal transition that is significant in the malignant progression to metastasis.
LIV-1 is expressed in numerous cancers with the greatest prevalence and expression level in prostate, breast and melanoma.
LIV-1 has also been connected to malignant progression to metastasis and is related to lymph node involvement in breast cancer.
Ladiratuzumab vedotin is an ADC made of a humanized anti-LIV-1 monoclonal antibody conjugated to the microtubule-disrupting agent MMAE through a protease-cleavable linker with Seattle Genetics’ proprietary technology.
In a 1/2 phase clinical trial, the combination of Ladiratuzumab vedotin and Keytruda, a PD-1 inhibitor, obtained an objective response rate of 54% in patients with metastatic and unresectable TNBC.
CA6
CA-6 is a perfect ADC target that has been selectively expressed in solid tumors. SAR566658 includes DM4 and an antibody targeting CA6. In a phase I clinical trial including 114 patients, 60% of patients in the 190 mg/m2 and 90 mg/m2 dose groups experienced tumor regression on days one and eight.
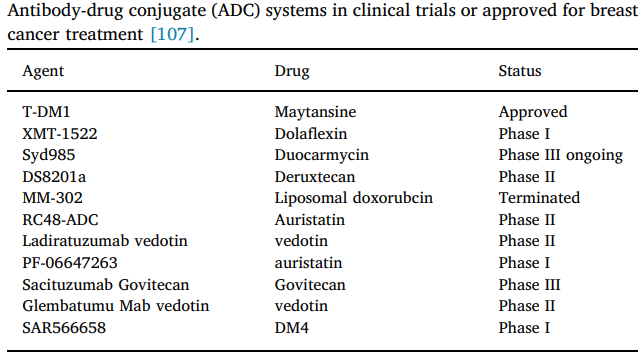
Figure 3. ADCs that have been approved or are in the clinical stage for the treatment of breast cancer (data from literature). Image Credit: ACROBiosystems
ACROBiosystems, a first-in-class manufacturer of recombinant proteins, has come up with a range of breast cancer-related target proteins with high purity, bioactivity, comprehensive species, and label, like VEGF/EGFR/TROP-2/LIV-1/GPNMB, which can completely aid the development of breast cancer-related drugs.
Product list
- Comprehensive products: 10+ breast cancer-related target proteins, like VEGF/EGFR/TROP-2/LIV-1/GPNMB; Immune checkpoint proteins like PD-1/PD-L1
- Stringent quality control: SDS-PAGE/MALS verifies the purity and SPR/BLI/ELISA verifies the bioactivity
- Different species and tags: Human, Mouse, Cynomolgus; Rat, Rabbit; His, Fc, His and Avi, tag-free, etc.
- The experimental protocol is available for free
Product List. Source: ACROBiosystems
| . |
. |
. |
| EGFR |
VEGF |
FGFR |
| NOTCH |
TROP-2 |
GPNMB |
| LIV-1 |
CA6 |
PRLR |
Assay data
High purity verified by MALS
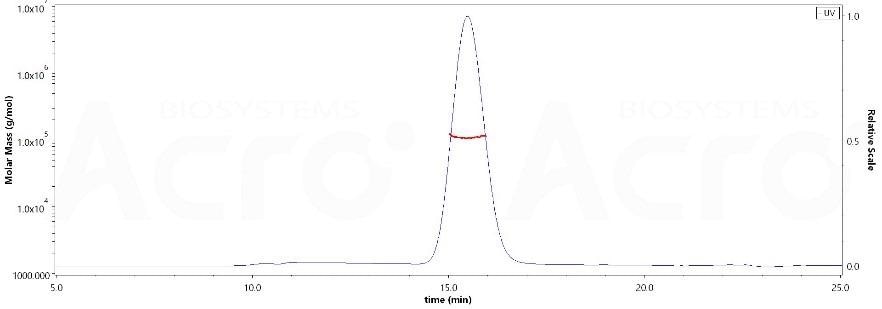
The purity of Human EGF R, His Tag (Cat. No. EGR-H5222) was over 90%, and the molecular weight of this protein is nearly 90–115 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
ELISA
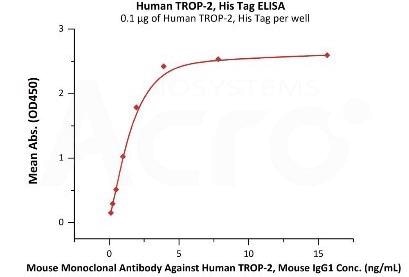
Immobilized Human TROP-2, His Tag (Cat. No. TR2-H5223) at 1 μg/mL (100 μL/well) can adhere Mouse Monoclonal Antibody Against Human TROP-2, Mouse IgG1 with a linear range of 0.1–2 ng/mL (QC tested). Image Credit: ACROBiosystems
SPR
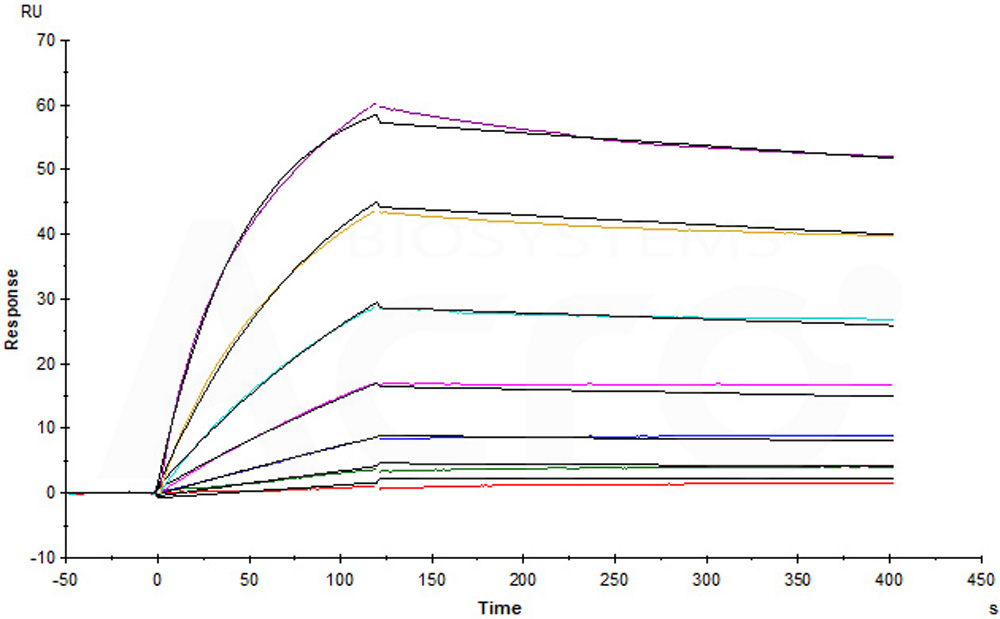
Anti-LIV-1 mAb immobilized on CM5 can attach Human LIV-1, Fc Tag (Cat. No. LV1-H5254) with an affinity constant of 1.74 nM as determined in an SPR assay (Biacore T200) (Routinely tested). Image Credit: ACROBiosystems
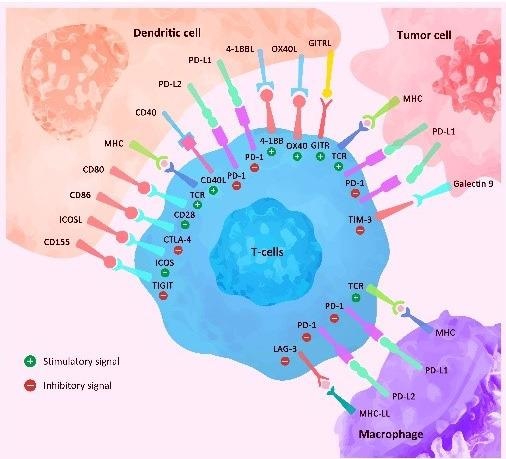
Image Credit: ACROBiosystems
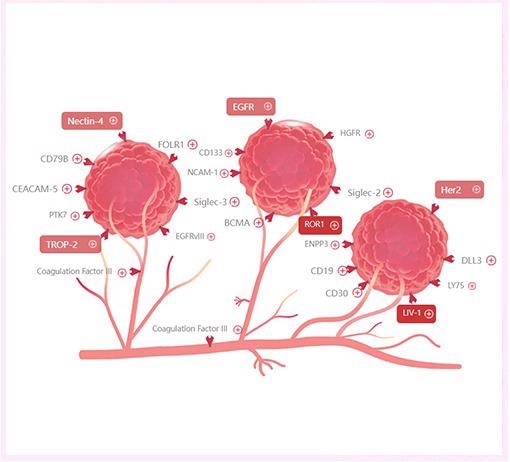
Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.