The healthcare company Abbott transformed the testing landscape of the United States. On 27th August, 2020, the company reported the acquisition of FDA authorization to distribute a new, pioneering type of test: a test that will cost just $5 each.
The Trump administration has subsequently declared that it will purchase 150 million of Abbott’s tests. For comparison, states have reported less than 75 million tests total over the past six months.
Abbott’s test is a kind of antigen test. Compared to the PCR test, it is believed to be quicker, more convenient, and costs less. Furthermore, in comparison to the antibody test, using the antigen test makes it possible to detect the virus in those who carry but don’t display symptoms – additionally, this is not restricted to the window of onset.
The antibody pairs are the vital reagents for antigen test kits. High-quality pairs of antibodies can help enhance specificity and sensitivity. Potentially, the sensitivity of the antigen test could be a match for the excellent sensitivity of the PCR test.
However, for the development of the antigen test, scientists are currently facing a dilemma regarding the best method of selection for an appropriate sample for the assay development. It is clear that the better sample is the patient's nasopharyngeal swab but due to biosafety reasons, it can be difficult to obtain access to patient samples early in the discovery stage.
So, a majority of the scientists utilize recombinant antigen proteins as spiked in samples for the assay development. Yet, this technique throws up inadequacies as some of the recombinant proteins may not best represent the structure of the virus. Moreover, the lysis of the swab samples will cause undesirable breakage of the proteins, which is the recombinant protein is unable to replicate. In summary, the recombinant protein is inadequate when screening for the antibody pairs.
Case study
Two varying antibody pairs were tested utilizing recombinant proteins and inactive virus. As illustrated in Figure 1 below, the sensitivities differ.
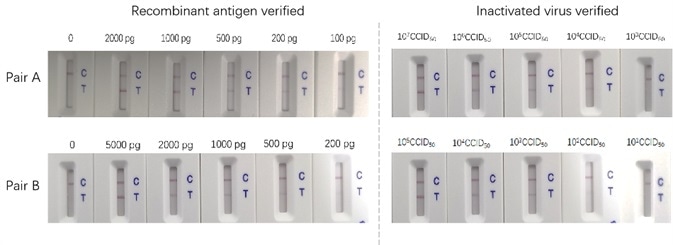
The most trusted way to assess an antigen test is conducted by utilizing an inactive virus for the assay development. For the purpose of supporting the IVD product development, ACRO prepared a series of high-grade recombinant protein reagents, in particular, the antibody pairs. ACRO’s antibody pairs have been tested and proven using inactivated virus samples. The sensitivity of the sandwich ELISA can be as low as 12 pg/ml. The titer, which was resolved through our LFA assay, is as low as 100 CCID50.
These antibodies were suggested for antigen detection by a double antibody sandwich.
>>> Virus lysis buffer is now available for the development of an antigen detection kit.
Assay data
Verification study using inactivated virus
A 10-fold serial dilution to the inactive virus samples was completed using lysis buffer (Cat. No. LY-13) from 105 CCID50 to 10 CCID50. Consequently, using ACRO anti-nucleocapsid antibody pairs, 100 ul of every sample was added to the LFA test stripe developed. Readouts were then taken at 15 minutes (+ clear line, +? vague line):
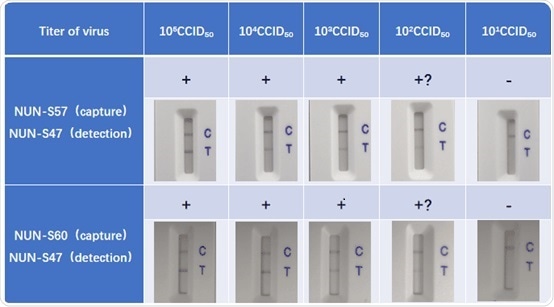
The assay was then repeated and the subsequent results were consistent, which demonstrates the reproducibility and high-quality ACRO anti-nucleocapsids antibody pairs.
High sensitivity of 12 pg/mL (ELISA assay)
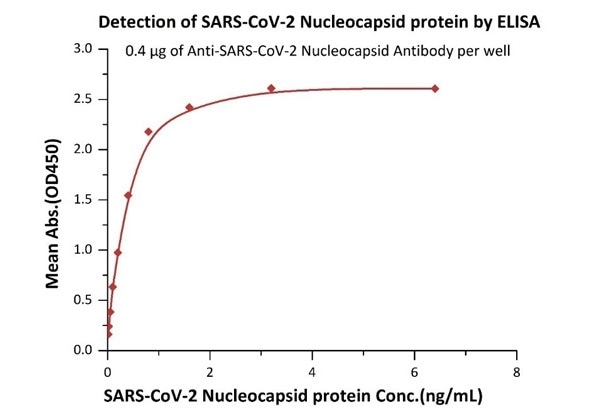
Figure 1. Immobilized Anti- SARS-CoV-2 Nucleocapsid antibody, mouse Mab (Cat. No. NUN-S46) at 4 μg/mL, add increasing concentrations of SARS-CoV-2 Nucleocapsid protein and then add Biotinylated Anti- SARS-CoV-2 Nucleocapsid antibody, mouse Mab (Cat. No. NUN-S47). Detection was performed using HRP-conjugated streptavidin with a sensitivity of 12 pg/mL. Image Credit: ACROBiosystems
Super affinity of 0.01 nM level (BLI assay)
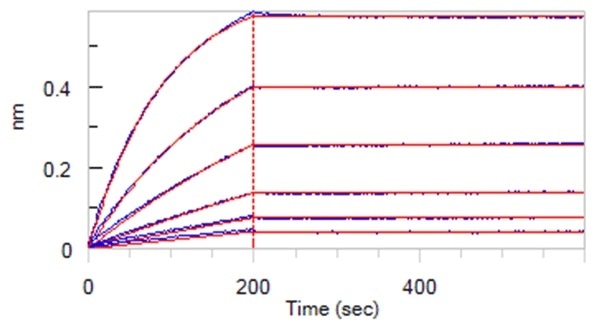
Figure 2. Anti-SARS-CoV-2 Nucleocapsid Antibody, Mouse MAb(Cat. No. NUN-S47) can bind SARS-CoV-2 Nucleocapsid ProteinCat. No. NUN-C5227) with an affinity constant of 24.7 pM determined in BLI assay (ForteBio Octet Red96e). Image Credit: ACROBiosystems
Specific binding to SARS-CoV-2 Nucleocapsid protein
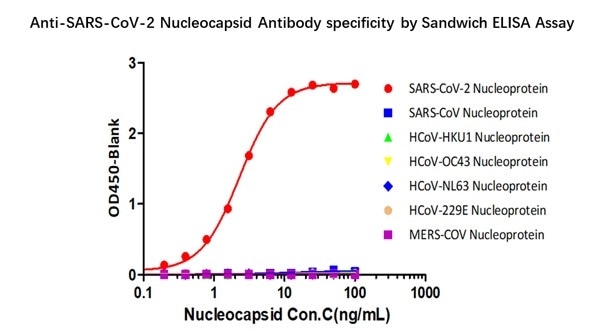
Figure 3. Demonstration of the specificity of Anti-SARS-CoV-2 N antibody pairs (Cat. No. NUN-S46 & Cat. No. NUN-S47) to the Nucleocapsid protein. Image Credit: ACROBiosystems
High batch-to-batch consistency
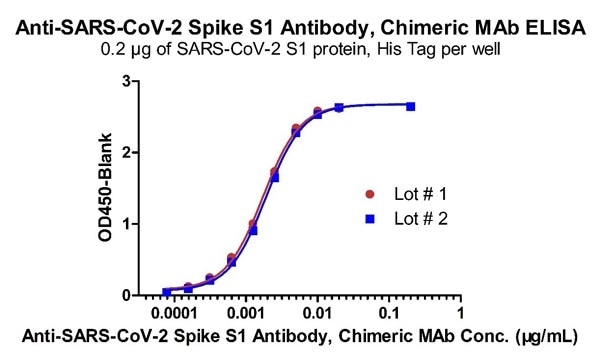
Figure 4. Demonstration of the Batch to Batch Consistency of Anti-SARS-CoV-2 Spike S1 Antibody, Chimeric Mab (Cat. No. S1N-M122) to the S1 protein. Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.