Eli Lilly officially launched its first dual GIP and GLP-1 receptor agonist injection in mainland China on 23 December 2024, attracting significant market attention.
The drug, MOUNJARO* (TIRZEPATID*), is a once-weekly treatment that delivers strong blood sugar control alongside notable weight loss. It has the potential to transform weight management therapy.
The highly competitive trillion-dollar metabolic disease market
Semaglutide has surged in popularity over the past two years, thanks to its remarkable effectiveness as a long-acting GLP-1 receptor agonist. High-profile endorsements, including from Elon Musk, have helped cement its reputation in the weight loss market as a so-called ‘miracle drug.’
This success has seen intense competition between pharmaceutical giants operating in the field of GLP-1 drugs. Barclays has predicted that the global weight loss drug market will reach $150 billion by 2030.

TIRZEPATID* is now available online in China, priced similarly to weight-loss Semaglutide. Image Credit: ACROBiosystems
On December 4, Eli Lilly announced that a head-to-head Phase 3 clinical trial had shown TIRZEPATID* to significantly outperform semaglutide in terms of weight loss efficacy.
This announcement saw Eli Lilly's stock rise by more than 2 % by market close, pushing its market capitalization to almost $790 billion. Considered the ‘strongest rival’ to Semaglutide, TIRZEPATID* leverages a dual-target mechanism of GIP and GLP-1 to achieve never-before-seen weight loss results.
TIRZEPATID* debuted on the Meituan platform on December 23, selling out in just three seconds on its launch night and highlighting strong enthusiasm in the Chinese market.
Platform data revealed that pre-launch reservations surged by 300 % daily, reflecting strong market recognition and high expectations.
The advantages of TIRZEPATID*
On 8 June 2024, Eli Lilly announced results from its Phase 2 SYNERGY-NASH trial of TIRZEPATID*, underscoring its potential as a treatment for patients with MASH.
The findings were first presented at the European Association for the Study of the Liver (EASL) Annual Meeting and simultaneously published in The New England Journal of Medicine (NEJM) in an article titled "TIRZEPATID* for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis."
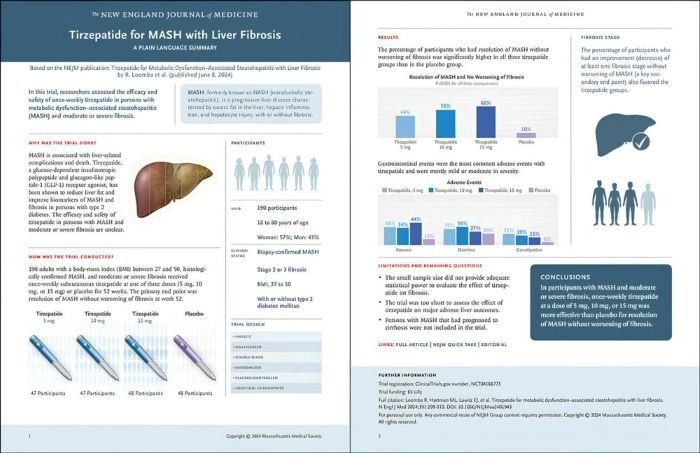
The study shows that TIRZEPATID* has a positive effect on MASH relief and the prevention and treatment of liver fibrosis 1. Image Credit: ACROBiosystems
The study revealed that the trial met its primary endpoint, confirming that after 52 weeks of treatment, 51.8 %, 62.8 %, and 73.3 % of patients receiving 5 mg, 10 mg, and 15 mg doses of TIRZEPATID* achieved MASH relief with no worsening of liver fibrosis, versus just 13.2 % in the placebo group (all doses, p<0.001).
ACROBiosystems’ contribution to the field
To meet the drug development needs of GIPR, GLP-1R, and GCGR agonists, and MASH-targeted agonists for THRA and THRB, ACROBiosystems has developed a portfolio of high-quality HEK293 cell-based products based on the signaling mechanisms of these molecules.
Human GLP-1R (Luc) HEK293 reporter cell, human GIPR (Luc) HEK293 reporter cell, human GCGR (Luc) HEK293 reporter cell, human THRA (Luc) HEK293 reporter cell, and human THRB (Luc) HEK293 reporter cell
These cell lines have been validated through receptor expression and functional activity. They are suitable for studies investigating agonist drug signaling functions, CMC quality control release applications, and cell-based drug activity assays and screening.
GLP-1R recombinant proteins, GIPR recombinant proteins, GCGR recombinant proteins, and GCGR recombinant protein (Detergent)
These proteins are expressed in human cells and verified using ELISA, SEC-MALS, SPR, and BLI. Each protein offers high activity, high purity, and excellent batch-to-batch consistency, making them ideally suited to antibody screening, immunology, and candidate drug function validation.
Full-length GLP-1R recombinant protein (Detergent) and full-length GCGR recombinant protein (VLP)
ACROBiosystems has developed full-length GLP-1R and full-length GCGR proteins using the ‘Membrane Masterpiece’ transmembrane target protein development platform.
This approach overcomes the challenges of low expression and the difficulty of maintaining native conformations typical of the seven-transmembrane proteins GLP-1R and GCGR.
The full-length GLP-1R protein’s biological activity has been validated via GLP-1R agonist binding, and the full-length GCGR protein has been verified via GCGR monoclonal antibody binding. Each of these proteins is ideally placed to help support the development of GLP-1R- and GCGR-targeted antibody drugs.
High, medium, and low expression HEK293/human GLP-1R stable cell line
Cell lines with different expression levels can support a range of applications, including early-stage antibody discovery, large-scale biopharmaceutical production, and functional studies of target proteins at natural expression levels.
GLP-1R antigens are stably expressed on the host cell membrane, effectively enabling antibody drug activity screening (cell-based binding and blocking) and the evaluation of CAR molecule killing activity.
Summary
All ACROBiosystems products are subjected to batch-by-batch quality control to verify key properties such as binding activity and purity. The company’s research team also provides free protocols with experimental parameters, saving its customers valuable R&D time.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond.
By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empowers scientists and engineers dedicated to innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.