When developing antibody-drug conjugates (ADCs), the linker structure significantly influences stability, homogeneity, cytotoxic potency, tolerability, and pharmacokinetics (PK).
Selecting the right linker is essential for optimizing the therapeutic potential and safety of ADCs. An ideal linker should remain stable in systemic circulation to prevent premature drug release yet enable efficient and specific payload release within the tumor microenvironment. Unfortunately, many existing linkers are prone to nonspecific release in non-tumor tissues, resulting in off-target toxicity.1
Creating novel linkers to avoid off-target toxicity is essential to improve therapeutic treatment with ADCs.
Linkers are generally classified as cleavable or non-cleavable based on their attachment mechanism. Over 80 % of clinically approved ADCs use cleavable linkers, such as Inotuzumab ozogamicin (Besponsa) and Brentuximab vedotin (Adcetris).2,3,4
Further types of cleavable linkers currently in use include cathepsin-cleavable, acid-cleavable, GSH-cleavable, Fe(II)-cleavable, and novel enzyme-cleavable linkers.5 Cathepsin-cleavable linkers have been particularly well studied and have been used in several approved ADCs.
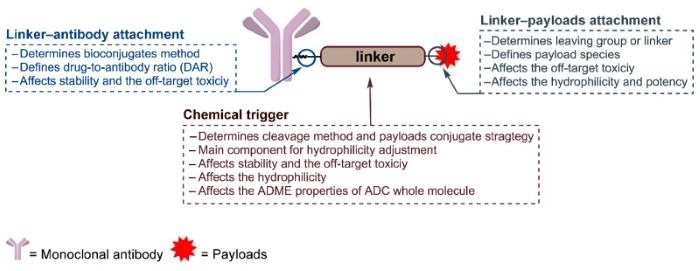
Figure 1. The general structure of an ADC and the roles of the chemical trigger, the linker‒antibody attachment and the linker‒payload attachment 5. Image Credit: ACROBiosystems
Cathepsin cleavable linkers
In 2017, Caculitan et al. discovered that the valine-citrulline (Val-Cit) linker shows broad sensitivity to various cathepsins, including cathepsin B, K, and L. However, only Cathepsin B is widely expressed in cancer cells, and its sensitivity to other cathepsins can lead to off-target toxicity in normal cells.
To enhance selectivity, Wei et al. designed a linker based on cyclobutane-1,1-dicarboxamide (cBu), which is primarily dependent on cathepsin B. Studies have shown that drug release linked via cBu-Cit was inhibited by over 75 % with a Cathepsin B inhibitor, while a Cathepsin K inhibitor showed minimal effect. ADCs with cBu-Cit linkers showed greater tumor inhibition in vitro compared to ADCs with Val-Cit linkers.
Research shows that even minor structural modifications to peptide linkers can significantly affect their performance. Zheng et al. developed a valine-alanine (Val-Ala) linker, demonstrating better hydrophilicity and stability than Val-Cit. Both Reid et al. and Salomon et al. have reported that ADCs incorporating (L,L)-dipeptide linkers show enhanced antitumor efficacy in multiple different settings.5
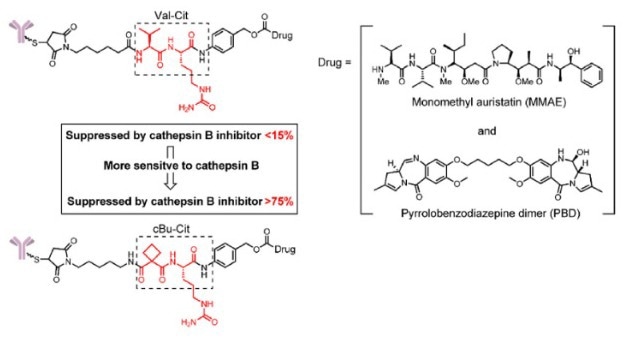
Figure 2. The structure of the cBu-Cit-PABC-containing ADCs 5. Image Credit: ACROBiosystems
Matrix metalloproteinase family cleavable linkers
Matrix metalloproteinase-2 (MMP-2) is highly expressed in numerous tumor tissues, including breast, cervical, and ovarian cancers.6 Studies suggest that MMP-2 plays a crucial role in promoting cancer cell metastasis and regulating tumor growth signaling pathways.7
MMP-2 can also cause degradation of extracellular matrix components in the tumor microenvironment, enhancing tumor invasion and metastasis. By exploiting these characteristics, researchers have developed various enzyme-reactive drug delivery systems.8-10 Unlike normal cells, the tumor microenvironment is characterized by hypoxia and acidity, providing an effective bio-trigger for controlled drug delivery in cancer therapy.11
Mu et al. introduced a novel ADC, FMSN-Dox-H2-AE01, utilizing human serum albumin (HSA)-shelled mesoporous silica nanoparticles as a biocompatible drug carrier. Potent chemotherapeutic agent doxorubicin is encapsulated within the mesoporous structure of this ADC, with an anti-EGFR antibody (AE01) linked for precise tumor targeting.12
The presence of HSA and antibodies on the particle surface enhances biocompatibility and prevents premature drug leakage. This system allows selective biodegradation triggered by MMP-2, ensuring specific cytotoxicity against cancer cells while minimizing effects on normal cells.
In vitro studies of this system have shown remarkable efficacy, with cancer cell mortality rates reaching approximately 85-90 %. Additionally, FMSN-Dox-H2-AE01 provides efficient control over drug release which is modulated by MMP-2 levels and pH conditions. These results highlight the potential of enzyme-responsive FMSN-Dox-H2-AE01 for cancer therapy.12
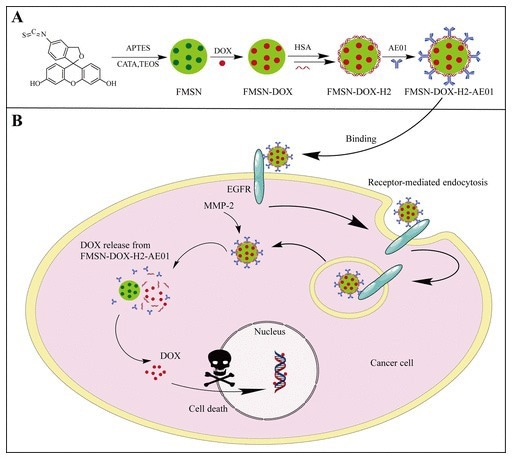
Figure 3. (A) Schematic description of anti-EGFR (AE01) and HSA functionalized mesoporous silica nanoparticles.(B) Mechanism diagram ofFMSN-DOX-H2-AE01. Image Credit: ACROBiosystems
Katrin et al. developed a proteolytically activatable ADC targeting IgM for treating IgM-positive B cell lymphoma. By integrating a masking unit derived from the IgM antigen into full-length antibodies, they engineered a sophisticated construct. The masking unit was fused with a synthetic linker containing dual-protease sites recognized by MMP-2/9 and matriptase, ensuring activation within the tumor microenvironment.
This design, based on a chicken anti-IgM antibody conjugated with MMAE, showed targeted binding, internalization, and controlled cytotoxic payload release, effectively inducing tumor cell death. The results from this study indicated that the masked CH2-aIgM had considerably reduced binding to IgM-positive cells compared to the unmasked version, which regained activity upon protease treatment.
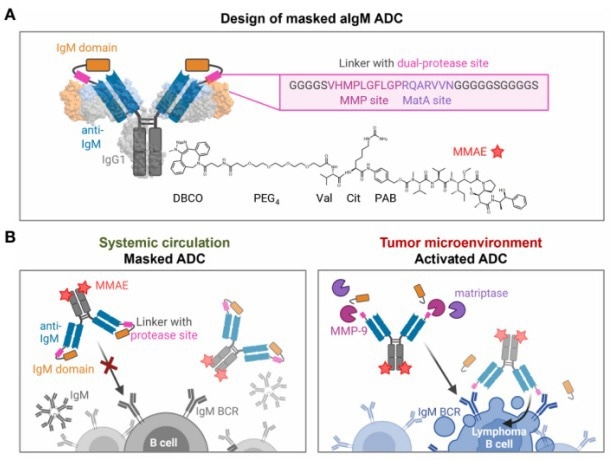
Figure 4. Design and mode of action of masked aIgM ADC. Image Credit: ACROBiosystems
Novel enzyme cleavable linkers
In addition to classical β-glucuronidase-cleavable linkers developed for ADCs, β-galactosidase, which is overexpressed in some tumors, also exhibits hydrolytic activity. Kolodych et al. introduced ADCs with a β-galactosidase-cleavable linker, which was rapidly hydrolyzed in vitro by 10 U/mL β-galactosidase.
Their ADC, linking trastuzumab and MMAE through this β-galactosidase-cleavable linker, showed a lower IC50 than Val-Cit-linked ADCs and Kadcyla. Further in vivo studies demonstrated significant tumor volume reductions of 57 % and 58 % in a xenograft mouse model at a dose of 1 mg/kg, outperforming Kadcyla at the same dose.14
Similarly, Bargh et al. introduced a sulfatase-cleavable linker that targets sulfatase, similarly to β-galactosidase in tumor cells. This linker exhibited high susceptibility to sulfatase enzymes and improved cytotoxicity and selectivity in HER2+ cells, in comparison to non-cleavable and Val-Ala ADCs.5
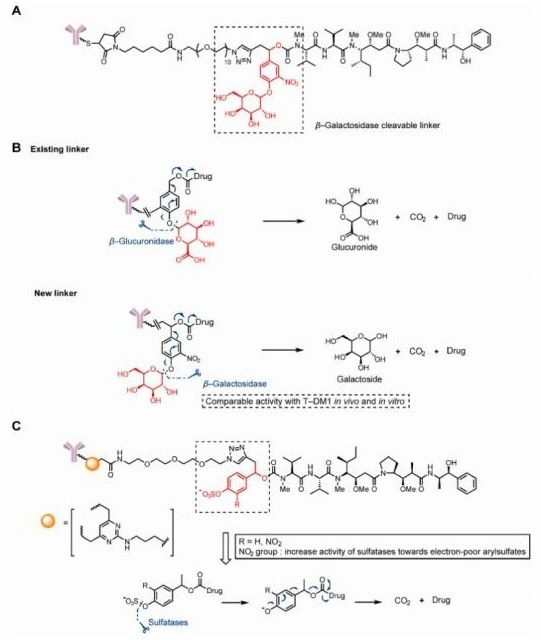
Figure 5. Structures of glycosidase- and sulfatase-cleavable triggers. (A)The structure of a β-glucuronidase-cleavable, linker-containing ADC. (B)Release mechanism of b-glucuronidase and β-glucuronidase-cleavable linkercontaining ADCs. (C)The structure and release mechanism of sulfatase-cleavable linker-containing ADCs. Image Credit: ACROBiosystems
ACROBiosystems has developed a series of specialized proteases for the screening and validation of ADC linkers, focusing on linker cleavage. These include Cathepsin B, Cathepsin L, Cathepsin S, MMP-2, MMP-7, MMP-9, β-glucuronidase, and β-galactosidase.
Product features
- Natural conformation: HEK293-expressed proteins ensure the natural structure.
- Robust and consistent enzyme activity: Ensuring reproducibility across batches.
- High purity: Verified by SDS-PAGE and SEC-MALS.
Additional features
- Analysis and characterization for ADCs: Conducting linker screening and stability evaluation.
- Assessment of drug release: Ensuring efficient intracellular payload delivery.
Source: ACROBiosystems
| Molecule |
Cat. No. |
Product Description |
| Cathepsin B |
CTB-H5222 |
Human Cathepsin B / CTSB Protein, His Tag (MALS verified) |
| Cathepsin B |
CTB-M52H9 |
Mouse Cathepsin B / CTSB Protein, His Tag (MALS verified) |
| Cathepsin L |
CAL-H52H3 |
Human Cathepsin L / CTSL1 Protein, His Tag (active enzyme) |
| Cathepsin L |
CAL-M52H3 |
Mouse Cathepsin L / CTSL1 Protein, His Tag (MALS verified) |
| Cathepsin S |
CTS-H52H9 |
Human Cathepsin S / CTSS Protein, His Tag (active enzyme, MALS verified) |
| MMP-9 |
MM9-C52H3 |
Cynomolgus MMP-9 Protein, His Tag (active enzyme) |
| MMP-9 |
MM9-H5221 |
Human MMP-9 Protein, His Tag (active enzyme) |
| MMP-9 |
MM9-H5229 |
Human MMP-9 Protein, His Tag (active enzyme) (MALS verified) |
| MMP-9 |
MM9-H52H9 |
Human MMP-9 (20-707) Protein, His Tag (active enzyme, MALS verified) |
| MMP-9 |
MM9-M52H1 |
Mouse MMP-9 (20-471) Protein, His Tag (active enzyme, MALS verified) |
| MMP-2 |
MM2-M52H9 |
Mouse MMP-2 (30-460) Protein, His Tag (active enzyme) |
| MMP-7 |
MM7-H5249 |
Human MMP-7 / PUMP1 Protein, His Tag (active enzyme) |
beta-Glucuronidase/
GUSB |
BEB-H52H3 |
Human beta-Glucuronidase/GUSB Protein, His Tag (active enzyme) |
beta-
Galactosidase-1 |
BG1-H52H3 |
Human beta-Galactosidase-1 Protein, His Tag (active enzyme) |
Enzyme Activity of Cathepsin B. Source: ACROBiosystems
| |
|
| Product |
Cat. No. CTB-H5222
Human Cathepsin B / CTSB Protein, His Tag (active enzyme) |
| Substrate |
Fluorogenic peptide substrate Z-LR-AMC |
| Enzyme Activity (pmol/min/μg) |
> 2,500 |
Enzyme Activity of MMP-9. Source: ACROBiosystems
| |
|
| Product |
Cat. No. MM9-H5221
Human MMP-9 Protein, His Tag (active enzyme) |
| Substrate |
Fluorogenic peptide substrate Mca-PLGL-Dpa-AR-NH2 |
| Enzyme Activity (pmol/min/μg) |
> 2,500 |
References
- Phuna, Z.X., et al. (2024). Antibody-drug conjugates: Principles and opportunities. Life Sciences, (online) 347, p.122676. https://doi.org/10.1016/j.lfs.2024.122676.
- Lyon, R. (2018). Drawing lessons from the clinical development of antibody-drug conjugates. Drug Discovery Today: Technologies, 30, pp.105–109. https://doi.org/10.1016/j.ddtec.2018.10.001.
- Hamann, P.R., et al. (2002). Gemtuzumab Ozogamicin, A Potent and Selective Anti-CD33 Antibody−Calicheamicin Conjugate for Treatment of Acute Myeloid Leukemia. Bioconjugate Chemistry, 13(1), pp.47–58. https://doi.org/10.1021/bc010021y.
- Perini GF, Pro B. Brentuximab vedotin in CD30þ lymphomas. Biol Ther 2013;3:15e23.
- Su, Z., et al. (2021). Antibody–drug conjugates: Recent advances in linker chemistry. Acta Pharmaceutica Sinica B, (online) 11(12), pp.3889–3907. https://doi.org/10.1016/j.apsb.2021.03.042.
- Niedzwiecki (2010). In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncology Reports, 23(3). https://doi.org/10.3892/or_00000675.
- Kessenbrock, K., Plaks, V. and Werb, Z. (2010). Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell, 141(1), pp.52–67. https://doi.org/10.1016/j.cell.2010.03.015.
- Chen, W.-H. et al. (2014) 'MMP-2 responsive polymeric micelles for cancer-targeted intracellular drug delivery,' Chemical Communications, 51(3), pp. 465–468. https://doi.org/10.1039/c4cc07563c.
- Peng, Z.-H. and Jindřich Kopeček (2015). Enhancing Accumulation and Penetration of HPMA Copolymer–Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. Journal of the American Chemical Society, 137(21), pp.6726–6729. https://doi.org/10.1021/jacs.5b00922.
- Taghizadeh, B., et al. (2014). Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Delivery, 22(2), pp.145–155. https://doi.org/10.3109/10717544.2014.887157.
- Li, Y.-F., et al. (2017). Heterodimers Made of Upconversion Nanoparticles and Metal–Organic Frameworks. Journal of the American Chemical Society, 139(39), pp.13804–13810. https://doi.org/10.1021/jacs.7b07302.
- Wu, H., et al. (2023). Constructed Tumor-Targeted and MMP-2 Biocleavable Antibody Conjugated Silica Nanoparticles for Efficient Cancer Therapy. ACS Omega, 8(14), pp.12752–12760. https://doi.org/10.1021/acsomega.2c07949.
- Schoenfeld, K., et al. (2023). Conditional activation of an anti-IgM antibody-drug conjugate for precise B cell lymphoma targeting. Frontiers in immunology, (online) 14, p.1258700. https://doi.org/10.3389/fimmu.2023.1258700.
- Kolodych, S., et al. (2017). Development and evaluation of β-galactosidase-sensitive antibody-drug conjugates. European Journal of Medicinal Chemistry, (online) 142, pp.376–382. https://doi.org/10.1016/j.ejmech.2017.08.008.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.