Transforming growth factor-beta (TGF-β) is a dynamic cytokine that is part of the transforming growth factor superfamily.
The TGF-β signaling pathway is connected to numerous cellular processes in both the developing embryo and the adult organism, including cell growth, cell differentiation, cellular homeostasis, apoptosis and other cellular functions.
TGF-β signaling in cancer is a complicated process. While TGF-β can facilitate the suppression of tumor formation, conversely, it can also foster it.
In healthy cells and early-stage cancers, TGF-β signaling suppresses tumor formation by impeding cell growth and apoptosis. However, in progressive cancers, TGF-β signaling receives a variety of tumor-promoting effects through specific adaptation or signaling pathways bypass.
There are two mechanisms to explain the tumor-promoting effects of TGF-Β:
1. The tumor utilizes TGF-β to encourage the epithelial mesenchymal transition (EMT), thereby amplifying the migration, infiltration, invasion and extravasation of the tumor cells.
2. Through paracrine signaling, TGF-β can assist in the formation of the tumor microenvironment (TME), switching on cancer-associated fibroblasts (CAF), encourage angiogenesis, generate extracellular matrix (ECM), impede anti-tumor immune response and ultimately foster tumor metastasis (Figure 1).
Figure 1. Tumor-promoting and suppressing effect of TGF-β. Image Credit: ACROBiosystems
The TME is the environment surrounding a tumor, including the bordering blood vessels, fibroblasts, immune cells, signaling molecules and ECM. TEM is crucial for tumor immune escape. It generates a barrier to shield the tumor from the immune system and eventually leads to tumor metastasis.
Therefore, TME is vital for the development of a tumor and is one of the principal issues that need to be addressed in tumor clinical treatment.
The TEM is induced by TGF-β through the following steps:
1. TGF-β activates/differentiates fibroblasts, mesenchymal stem cells, epithelial tumor cells, adipose tissue-derived stem cells and endothelial cells (Figure 2A). The fibroblasts are critical elements of the TME.
They encourage immune escape and angiogenesis by dispensing ECM and cytokines. Endothelium cells are situated on the surface of blood and lymphatic vessels. Blood vessels feed tumors by supplying blood/oxygen/nutrients, eliminating waste and limiting the entry and exit of immune cells and other substances.
TGF-β acts directly and indirectly on endothelial cells to foster angiogenesis and migration in TME (Figure 2b).
2. TGF-β represses the immune system by limiting the function of the immune cell populations in the tumor microenvironment (TME), which is spotted with a diverse range of innate and adaptive immune cells.
- TGF-β subdues tumor adaptive immunity by principally impeding T cell activation, differentiation, proliferation and migration (Fig. 2C). TGF-β typically inhibits the differentiation of primary CD4 + T cells into various effector subtypes, but it can prompt the transfiguration of primary T cells into regulatory T cells, which have an active immunosuppressive role.
- TGF-β prevents the activation and maturation of cytotoxic CD8 + T cells by impeding the conversion of tumor antigens and the expression of DCs. TGF-β also limits the proliferation of CD8 + T cells by preventing the expression of IFNγ and IL2.
- In addition, TGF-β encourages the expression of antigen-induced programmed cell death protein 1 (PD-1) in CD8 + T cells, which results in T cell failure.
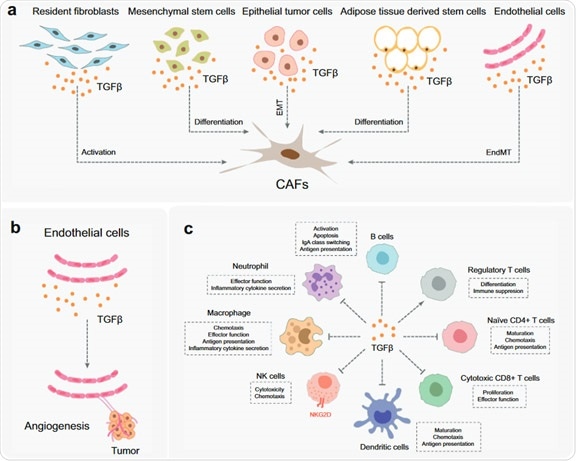
Figure 2. The function of TGF-β in TEM. Image Credit: ACROBiosystems
Since TGF-β is implicated in numerous aspects of tumor genesis and development, the suppression of TGF-β signal transduction produces many research targets for anti-tumor therapy, which has become a rapidly developing research field of new anti-cancer therapies.
Cancer cells often break free from TGF-β-induced cell suppression responses, which is why, in many cases, cancer patients show recurrent symptoms subsequent to chemotherapy, targeted therapy, or radiation.
The response of a single treatment regimen is restricted. Yet, the development of combination therapy has become widely accepted.
Utilizing an anti- TGF-β drug in parallel with one of the following treatments, including immune checkpoint inhibitors (such as PD-1/PD-L1 antibodies), radiation therapy, cytotoxic drugs and cancer vaccines.
In accordance with the clinical data, the anti-tumor effect of the combination of PD-L1 antibody and TGF-β antibody is more efficient than that of single-drug treatment (Table 1). Yet, the anti-cancer mechanism of TGF-β inhibitors is still not fully understood.
Table 1. The combination of PD-L1 and TNF- β antibodies in clinical trials. Source: ACROBiosystems
| Drug name |
The highest R&D status worldwide |
Active
company |
Active Indications |
target |
Mechanism |
| PM-8001 |
phase II
clinical trials |
Pumis Biotechnology
(Zhuhai)Co., Ltd. |
solid
tumor |
PD-L1
TGF-β |
Programmed death-ligand 1 inhibitors (PD-L 1 Inhibitor); Transforming growth factor beta inhibitors (TGF-β Inhibitor) |
| HBM-7015 |
Preclinical development |
Harbour BioMed |
solid
tumor |
TGF-β1
PD-L1 |
Transforming growth factor beta 1 inhibitors (TGF-β 1 Inhibitor); Programmed death-ligand 1 inhibitors (PD-L1 Inhibitor) |
Further developing the understanding of the interaction between the human body and TGF-β signaling is the crucial element to improving clinical efficacy in the future.
TGF-β signal transduction has garnered significant attention in scientific and clinical studies due to its key role in tumor immune escape. A comprehensive understanding of the mechanism of TGF-β can help enhance the tumor treatment plans in clinical practice and offer a new direction of cancer drug development across the industry.
ACROBiosystems developed a series of TGF-beta 1, Latent TGF-beta 1, TGF-beta 3, TGF-beta RII proteins independently and their biological activity has been validated by numerous technologies, including Cell-based assay and ELISA with excellent feedback from customers.
The relevant protocol is supplied for free in order to shorten the R&D cycle.
Product list
Table 2. Source: ACROBiosystems
| Molecule |
Cat. No. |
Product Description |
| TGF-beta 1 |
TG1-H4212 |
ActiveMax® Human TGF-Beta 1 / TGFB1 Protein, Tag Free |
TG1-H8217 |
Biotinylated Human TGF-Beta 1 / TGFB1 Protein, Avitag™ |
TG1-M5218 |
Mouse TGF-Beta 1 / TGFB1 Protein |
| TGF-beta RII |
TG2-H5252 |
Human TGF-beta RII / TGFBR2 Protein, Fc Tag |
TG2-H82E4 |
Biotinylated Human TGF-beta RII / TGFBR2 Protein, His, Avitag™ |
TG2-H82F6 |
Biotinylated Human TGF-beta RII / TGFBR2 Protein, Fc, Avitag™ (MALS verified) |
TG2-H52H5 |
Human TGF-beta RII / TGFBR2 Protein, His Tag (MALS verified) |
TG2-M82H5 |
Biotinylated Mouse TGF-beta RII / TGFBR2 Protein, His, Avitag™ |
LAP
(TGF-beta 1) |
LAP-H5245 |
Human LAP (TGF-beta 1) Protein, His Tag |
LAP-H82Q6 |
Biotinylated Human LAP (TGF-beta 1) Protein, His, Avitag™ |
| Latent TGF-beta 1 |
TG1-H524x |
Human Latent TGF-beta 1 / TGFB1 Protein, His Tag |
TG1-H82Qb |
Biotinylated Human LAP (TGF-beta 1) Protein, His, Avitag™ |
TG1-C5243 |
Cynomolgus Latent TGF-beta 1 (C33S) Protein, His Tag |
| TGF-beta 3 |
TG3-H8218 |
Biotinylated Human Latent TGF-beta 3 / Latent TGFB3 Protein, Avitag™ |
Assay data
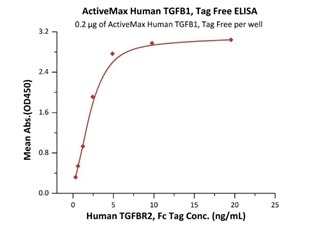
Immobilized ActiveMax® Human TGFB1, Tag Free (Cat. No. TG1-H4212) at 2 μg/mL (100 μL/well) can bind Human TGFBR2, Fc Tag (Cat. No. TG2-H5252) with a linear range of 0.3-5 ng/mL (QC tested). Image Credit: ACROBiosystems
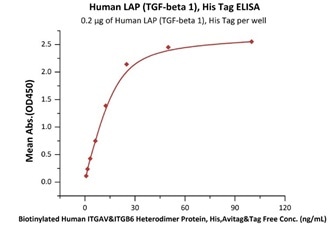
Immobilized Human LAP (TGF-beta 1), His Tag (Cat. No. LAP-H5245) at 2 μg/mL (100 μL/well) can bind Biotinylated Human ITGAV&ITGB6 Heterodimer Protein, His,Avitag&Tag Free (Cat. No. IT6-H82E4) with a linear range of 0.8-25 ng/mL (QC tested). Image Credit: ACROBiosystems
Cell based assay
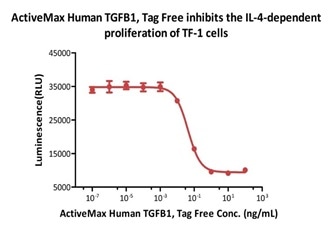
ActiveMax® Human TGFB1, Tag Free (Cat. No. TG1-H4212) inhibits the IL-4-dependent proliferation of TF-1 cells. The ED50 for this effect is 0.43-0.91 ng/mL (Routinely tested). Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.