Developing therapeutic antibody drugs is a lengthy, highly explorative process. There are many potential pitfalls, challenges, and risks during drug development, which can significantly impact the cost and timescale of research, as well as potential issues with drug efficacy and safety.
The level of uncertainty in therapeutic antibody drug development can be reduced through research and development of structurally defined and high-quality recombinant proteins. These proteins can be used for quality control and screening purposes.
Bispecific antibody drugs
A bispecific antibody drug (bsAb) is a second-generation antibody cancer therapeutic. First developed in the 1960s, screening and quality control are essential for this novel drug class.
The first bispecific antibody drugs were produced using thiol-redox methods to couple the Fab regions of two different-specificity antibodies. Kohler and Milstein developed hybridoma technology in 1975, which kickstarted a revolution in antibody therapies.
Following this key development, the field of antibody engineering accelerated. Developments, including asymmetric antibody pairing methods and single-chain variable fragments, were introduced. Today, bsAb development is seeing significant research focus, with the pharmaceutical pipeline increasing exponentially.
To date, over 250 drugs that target different kinds of cancer have entered preclinical trials. Alongside different bsAb engineering techniques developed in recent years, the mechanism of action has evolved.
Quality controls that evaluate the efficacy of final therapies must also consider the mechanisms of action. This is essential to ensure the comprehensive testing of these new therapeutics. To achieve this, quality control tools such as recombinant proteins must be consistent and high quality for a full evaluation of bsAbs.
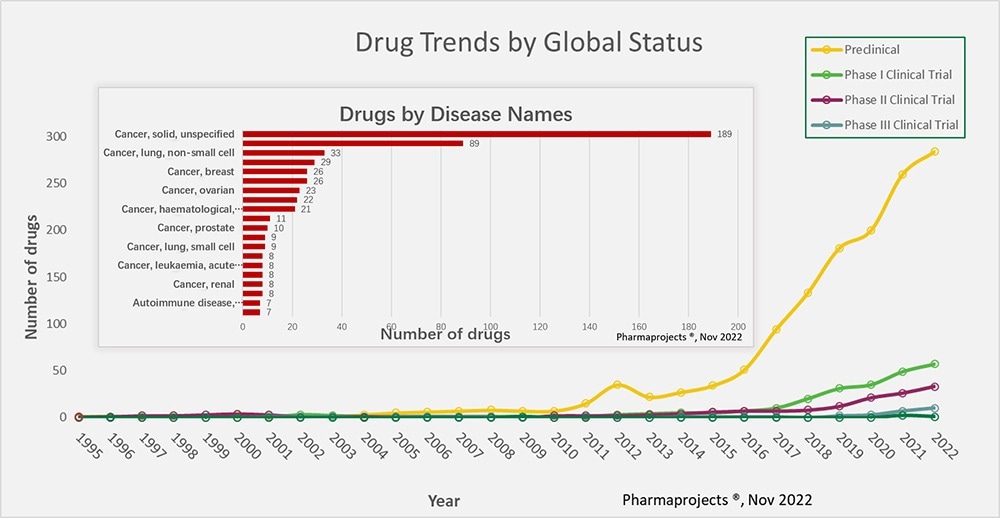
Figure 1. Trends from 1995 to 2022 for the development of bsAb drugs across the globe. (insert) bsAb target disease categories. Image Credit: ACROBiosystems
BsAbs mechanism of actions
Due to the various mechanisms of actions in BsAbs, they have demonstrated significant clinical promise. One common mechanism of action in bsAbs is their action as a cell engager, where they function as a bridge between two or more cells, bringing them closer to each other.
Other mechanisms of action include co-factor mimetics, where the drug binds to co-factors, initiating relevant signaling cascades, and receptor inhibition/activation, where they bind to multiple receptors on the same cell, enhancing affinity binding and drug effects,
Another mechanism of action is “piggy-backing”, where one side targets high-affinity receptors, leveraging a more favorable position for the other side of the bispecific antibody drug. This mechanism allows bsAb drugs to cross the blood-brain barrier, targeting low-affinity receptors that typically do not bind to monoclonal antibodies.
The dual-specificity of bispecific antibody drugs has allowed an expansion of possible mechanisms of action, which enhances the potential of this novel class of drugs as potential cancer therapeutics, providing clinical researchers with a new, powerful toolkit in the fight against cancer.
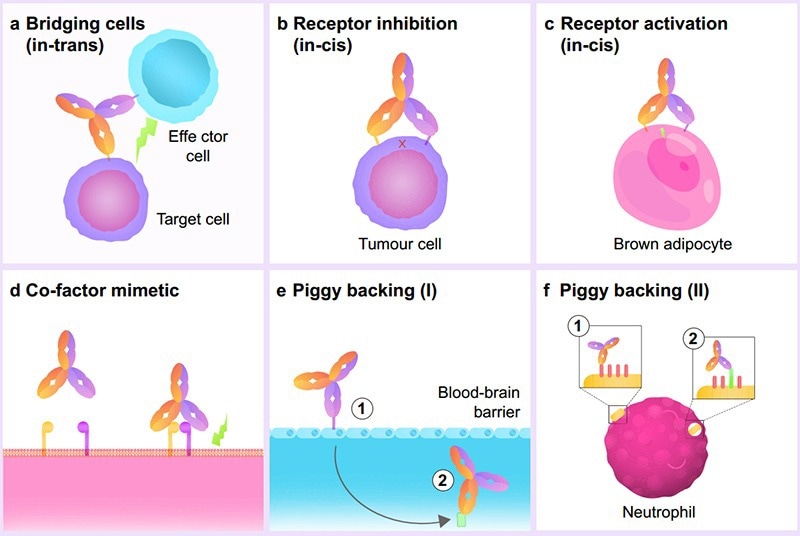
Figure 2. BsAbs fall into six subtypes based on their engineered biochemical activity. Image Credit: ACROBiosystems
First, recombinant protein characterization and purity was performed to ensure appropriate conformation and quality. SDS-PAGE and SEC-MALS were performed on the CD3δ/CD3ε heterodimer, identifying more than 90% purity and the correctly formed heterogeneous complex. To evaluate bioactivity, SPR and ELISA studies were performed using a human CD3 antibody (OKT3) and Human CD3E&CD3D heterodimer protein.
Quality control of CD3-targeting bass
CD3-targeting bispecific antibody drugs are a type of T-cell engager. The mechanism of action in these types of bsAb is the bridging cell action. The research into these cancer therapeutics is developing rapidly, with various types of bispecific antibody drugs approved and in the pipeline.
CD3 is a type of protein complex and T-cell co-receptor. It activates both T-helper cells and T-cells, which have cytotoxic action. Bispecific antibody drug binds to tumor-associated antigens and T cells, providing a bridging action that initiates tumor cell death.
This cytotoxic action is irrespective of T cell receptor specificity, peptide antigen presentation, or co-stimulation. Cytokine secretion is stimulated by T cell activation due to CD3 bsAbs recruiting additional immune system helper cells and enhancing anti-tumor activity.
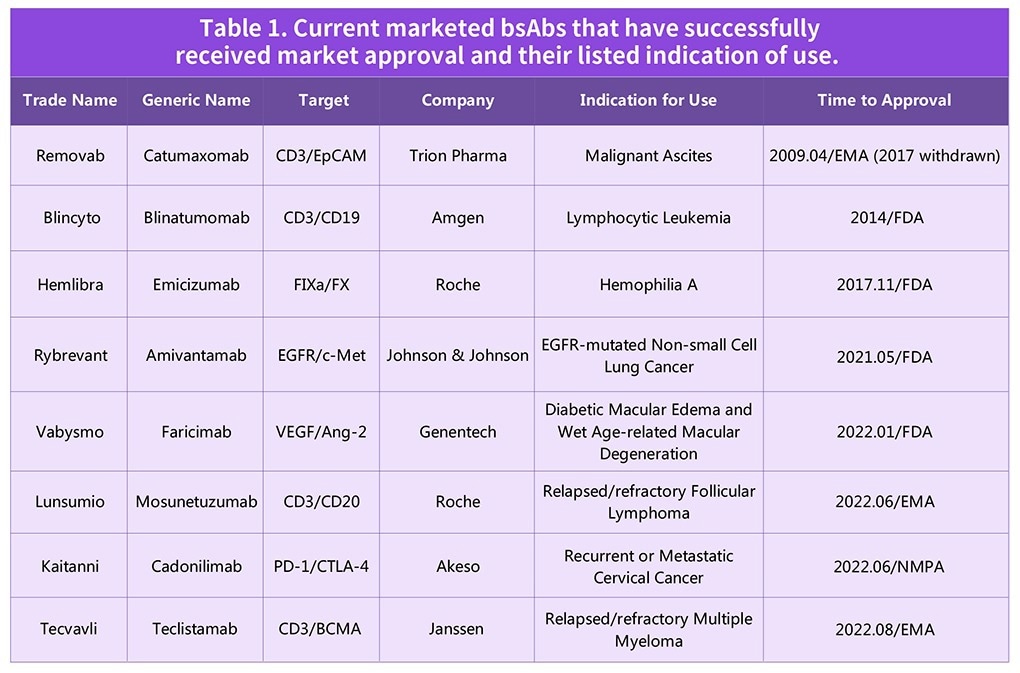
Table 1. Current marketed bsAbs that have successfully received market approval and their listed indication of use. Source: ACROBiosystems
Using well-defined, high-quality CD3 proteins in quality control is crucial for the significant number of CD3-targeting bispecific antibody drugs currently in the pipeline. However, there is a key issue that presents a critical challenge: the complex nature of CD3’s recombinant expression.
CD3 has four distinct subtypes: CD3δ, CD3ε, CD3γ, and CD3ζ. Several heterodimer pairs form the TCR-CD3 complex through the α/β chain of TCR.
CD3-targeted bsAbs usually target the heterodimer complex’s CR3ε subunit to activate T cell-mediated anti-tumor activity.
In recombinant expression, however, incorrect heterogenous complexes can be randomly formed by CD3δ and CD3ε. Thus, quality control can be extremely challenging in cancer therapeutics.
Verifying recombinant CD3 heterodimers for quality control
A comprehensive verification of produced recombinant proteins must be undertaken to address issues with CD3 protein expression inconsistencies in bsAbs. Verification methods include bioactivity studies, in-depth characterizations of protein structures, and batch-to-batch analyses.
Several current studies have employed a wide array of tests to characterize homogeneous CD3γ/CD3ε and CD3δ/CD3ε using recombinant proteins, including SDS-PAGE, SEC-MALS, SPR, and ELISA.
Pharmacokinetic analysis of T cell engagers has been evaluated using the CD3δ/CD3ε heterodimer in studies after these initial tests.
Studies have observed enhanced binding affinities between CD3 recombinant protein and OKT3. Batch-to-batch analysis ensures consistent bioactivity between different CD3 heterodimer combinations.
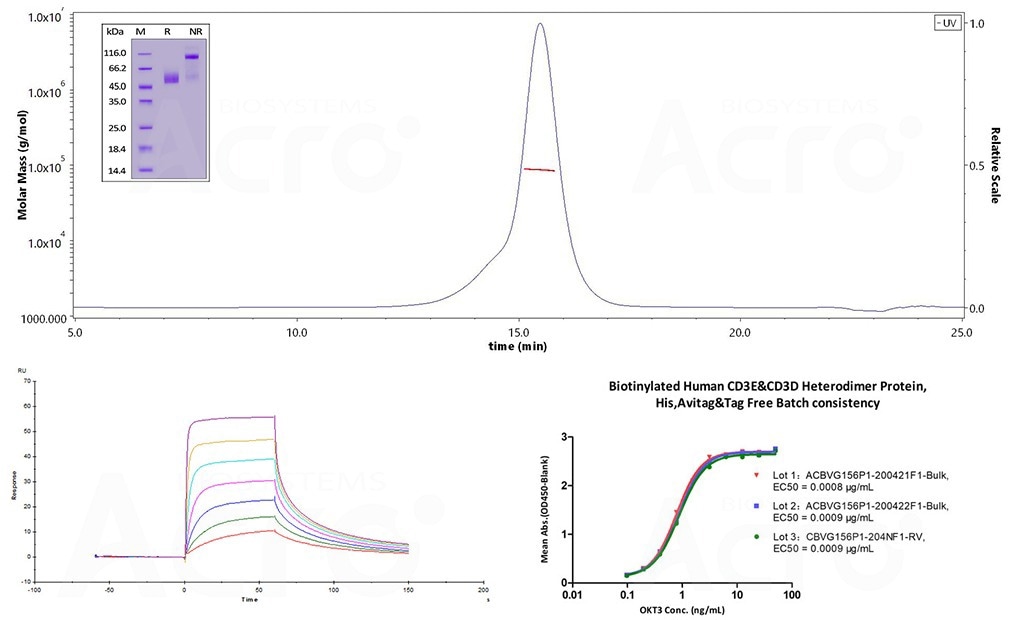
Figure 3. (Top) SDS-PAGE (insert) and SEC-MALS evaluation for structural homogeneity and purity. The purity of Human CD3E&CD3D Heterodimer Protein (Cat. No. CDD-H52Wa) is more than 90% using SDS-PAGE under reducing (R) and non-reducing(NR)conditions. The molecular weight is 80-90 kDa verified by SEC-MALS. (Bottom-left) SPR assay and (Bottom-right) ELISA were performed to evaluate the binding of Biotinylated Human CD3E&CD3D, His, Avitag & tag-free (Cat. No. CDD-H82W6) with anti-human CD3 antibody OKT3. The SPR assay identified an affinity constant of 22.5 nM with biotinylated human CD3 captured on a Biotin CAP-Series S sensor chip and evaluated on the Biacore T200. (Bottom-right) The linear range in the ELISA assay was identified to be 0.2 to 6 ng/mL. Lot differences were minimal, with EC50 differences between each lot less than 0.0001 ug/mL. Image Credit: ACROBiosystems
Studies have used the same CD3 protein in final ELISA studies post-protein quality evaluation. Thus, the quality of bsAb CD3 T-cell engagers can be comprehensively evaluated. One study immobilized BCMA onto the well-plate with 10% human serum spiked biotinylated CD3 recombinant protein.
CD3 x BCMA antibody-bound HRP conjugated streptavidin was detected in the study, with a sensitivity of 15 ng/mL. Therefore, bispecificity to both CD3 and BCMA was demonstrated in the study.
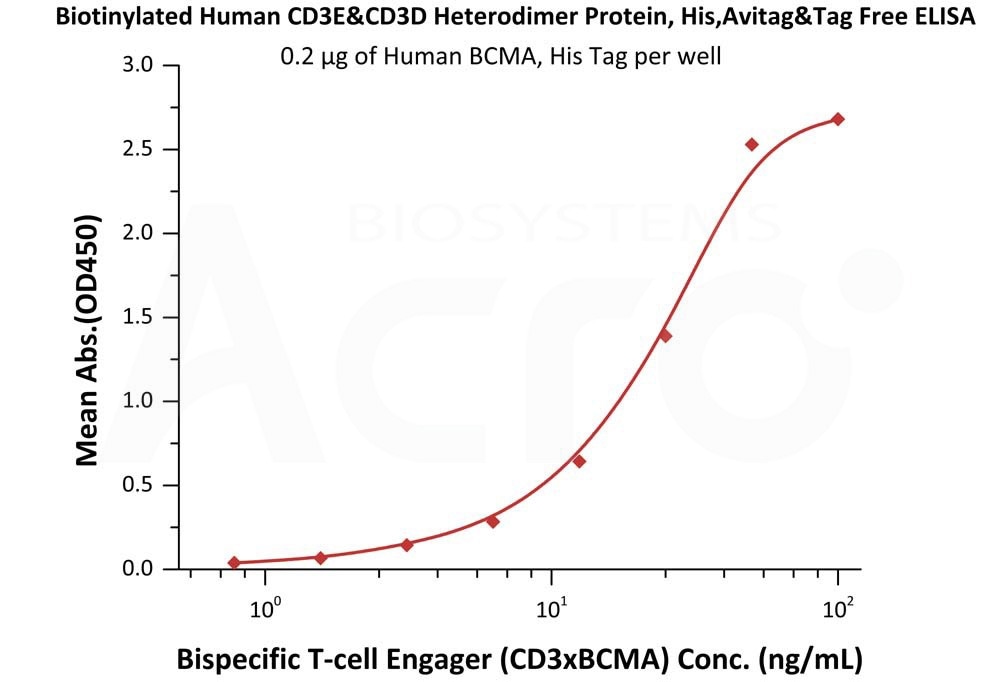
Figure 4. Immobilized Human BCMA, His Tag (Cat. No. BCA-H522y) was bound to a 96-well plate. Increasing concentrations of Bispecific T cell Engager (CD3 X BCMA) spiked in 10% human serum and Biotinylated Human CD3E&CD3D Heterodimer Protein, His,Avitag&Tag Free (Cat. No. CDD-H82W6) at 0.2 μg/mL was added to each well. Image Credit: ACROBiosystems
Summary
In novel, targeted cancer therapeutics, bispecific antibody drugs are relatively new, first developed in the 1960s, but the target of significant research attention in recent years.
Many companies are now accelerating the development of these innovative drugs from the concept phase to clinical trials.
Due to the rapidly evolving field of bispecific antibody drugs, quality control is critical. Recombinant proteins are a key reagent in bsAb studies, and ACROBiosystems provides high-quality, consistent, and bioactive recombinant proteins, including CD3. Contact the experts at ACROBiosystems today to find out more.
References and Further Reading
- Vafa O, et al. (2020) Perspective: Designing T-Cell Engagers With Better Therapeutic Windows. Frontiers in oncology
- Labrijn AF, J et al. (2019) Bispecific antibodies: a mechanistic review of the pipeline. Nature reviews. Drug discovery
- Krishnamurthy A, J. A, (2018) Bispecific antibodies for cancer therapy: A review. Pharmacology & therapeutics
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.