The 2020 meeting of the American Society of Clinical Oncology (ASCO) concluded just over a month ago. Due to the impact of the COVID-19 pandemic, the meeting was conducted virtually. The ASCO Annual Meeting, one of the largest clinical oncology conferences globally, operates as a platform for oncology professionals to exchange the latest advancements in the field of clinical oncology. This year, briefings around BCMA, TIGIT, CD47 as well as several other targets were shared.
Roche shared their data of the tiragolumab positive Phase II clinical trial, an anti-TIGIT monoclonal antibody, which attracted significant attention. Their result demonstrates a 67% decrease in the risk of deterioration or death caused by the disease (median PFS=not reached vs 3.9 months; HR=0.33, 95% CI: 0.15–0.72) with when compared with Tecentriq alone.
These positive results usher in the idea that blockade of TIGIT and PD-L1 may synergistically facilitate the reactivation of T-cells and strengthen NK cell anti-tumor activity.
TIGIT was originally detected in a genomic search for genes expressed particularly in T cells that possessed protein domain structures typical of potential inhibitory receptors. TIGIT satisfies the role of coinhibitory receptors to counteract the costimulatory role of CD226.
TIGIT contends with immunoactivator receptor CD226 (DNAM-1) for the same group of ligands: CD155 (PVR or poliovirus receptor) and CD112 (Nectin-2 or PVRL2). Both NK cells and T cells demonstrated the expression of TIGIT and it acts as a catalyst in their activation and maturation.
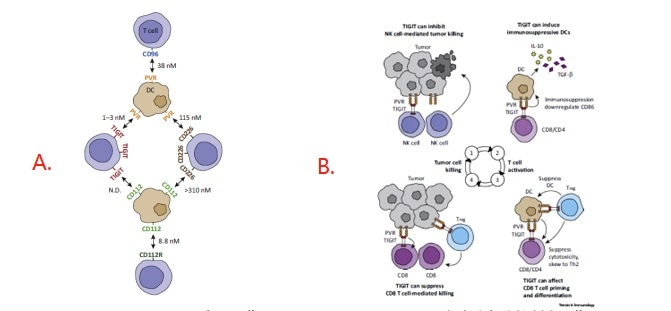
Figure 1. A: Expression and Interactions of PVR Family Members; B: Inhibition of the Cancer Immunity Cycle by TIGIT.
Emergent studies have revealed that blockade of TIGIT could have the capacity to supplement existing immunotherapies. TIGIT has shown excellent potentiality in preclinical models as a novel target for cancer immunotherapy and may work synergistically to broaden the activity of accepted checkpoint inhibitors, like anti-CTLA-4, anti-PD-1, etc. According to the record of the FDA, there are currently around 20 clinical trial studies focused on the target TIGIT.
Table 1. Selected TIGIT Targeted Research Summary.
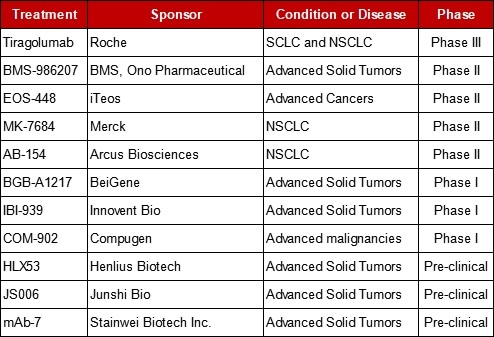
Roche has commenced its Phase III clinical trial of anti-TIGIT antibody tiragolumab. Besides Roche, there are several companies in the following suit. The first to get its NDA approved from CFDA is Innovent Bio for its TIGIT antibody IBI939. This antibody can directly merge with TIGIT to alleviate the exhaustion of T cells and NK cells, therefore, fostering the anti-tumor effect. Meanwhile, it is anticipated to synergistically increase the anti-tumor activity of PD-1/PD-L1 antibody drugs.
BeiGene has started a Phase 1a/1b clinical trial of BGB-A1217 in combination with tislelizumab for advanced solid tumors in China and Australia. As an increasing number of pharmaceutical companies join this competition, advancements in TIGIT targeted antibodies will become a trend in the near future.
ACROBiosystems has prepared a series of TIGIT recombinant proteins to facilitate the progression of antibodies and the screening of inhibitors. The products are certified as a homodimer by MALS and are appropriate for numerous applications during the drug development process.
Assay data
Homodimer verified by MALS
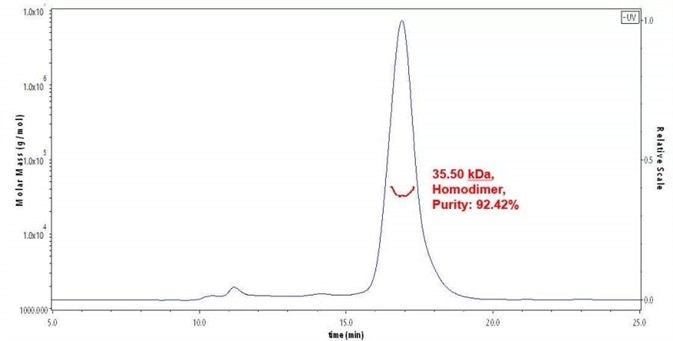
Figure 1. The purity of Human TIGIT Protein, His Tag (Cat. No. TIT-H52H3) was more than 90% and around 30-45 kDa verified by SEC-MALS.
Application in different phases of drug development
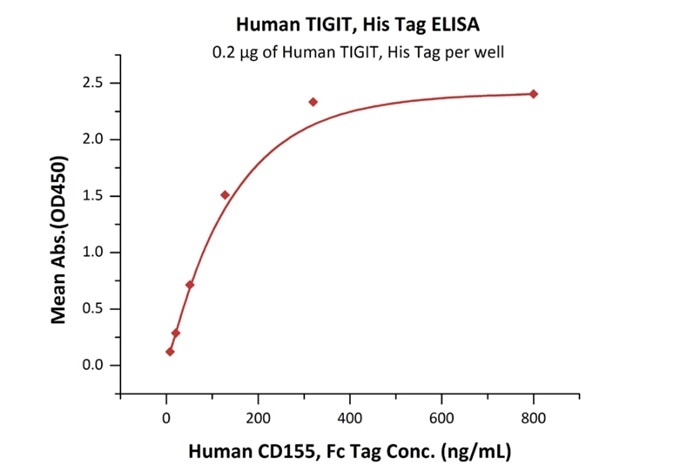
Figure 2. Immobilized Human TIGIT, His Tag Cat. No. TIT-H52H3) at 2 μg/mL (100 μL/well) can bind Human CD155, Fc Tag (Cat. No. CD5-H5251) with a linear range of 8-128 ng/mL.
TIGIT: CD155 inhibitor screening
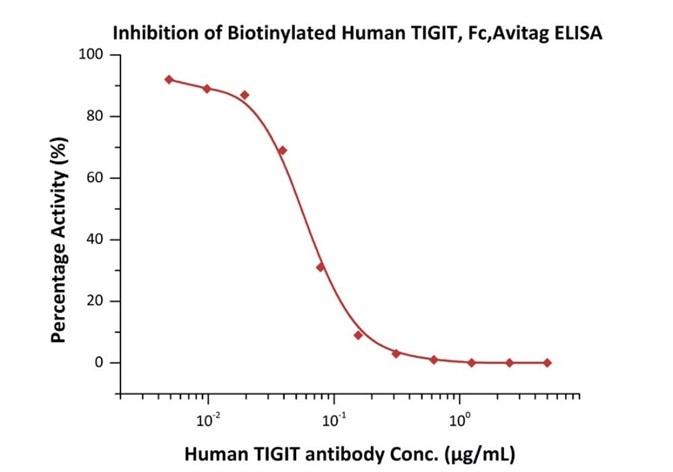
Figure 3. Serial dilutions of Human TIGIT Neutralizing antibody were added into Human CD155, Fc Tag (Cat. No. CD5-H5251): Biotinylated Human TIGIT, Fc, Avitag (Cat. No. TIT-H82F1) binding reactions. The half-maximal inhibitory concentration (IC50) is 0.06065 μg/mL.
BLI
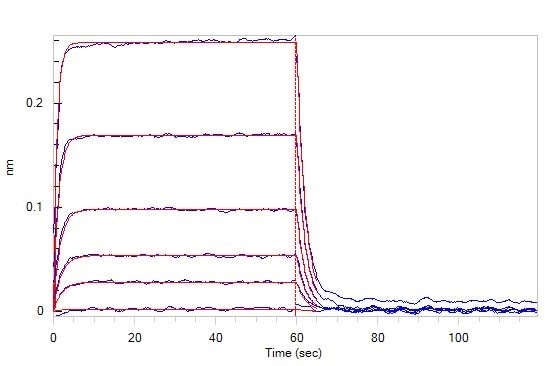
Figure 4. Loaded Human TIGIT, Fc Tag (Cat. No. TIT-H5254) on Protein A Biosensor, can bind Human CD155, His Tag (Cat. No. CD5-H5223) with an affinity constant of 0.98 μM as determined in BLI assay (ForteBio Octet Red96e).
SPR
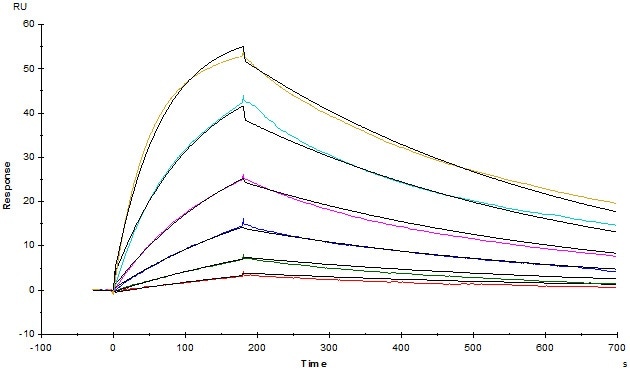
Figure 5. Anti-Human TIGIT MAb (Mouse IgG1) captured on CM5 chip via Anti-Mouse antibodies surface, can bind Human TIGIT, His Tag (Cat. No. TIT-H52H3) with an affinity constant of 3.93 nM as determined in an SPR assay (Biacore T200).
FACS
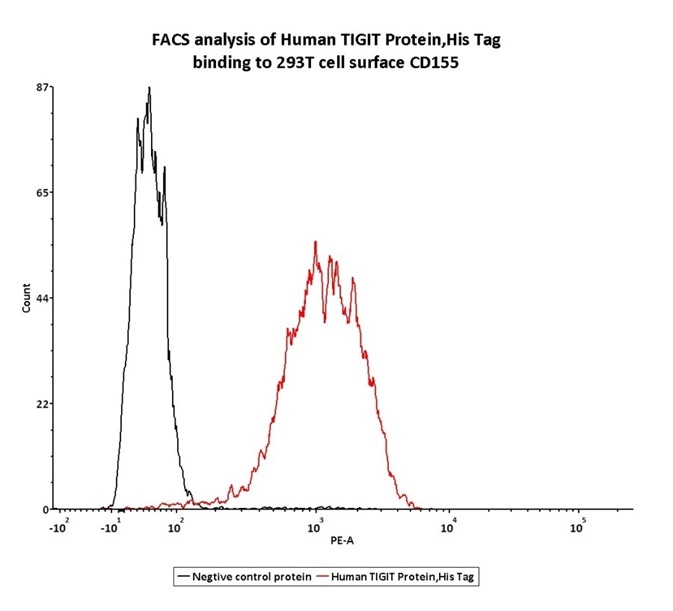
Figure 6. FACS assay shows that Human TIGIT, His Tag (Cat. No. TIT-H52H3) can bind to 293T cell overexpressing human CD155. The concentration of TIGIT used is 0.3 μg/mL.
Table 2. Product list.
Cat.
No. |
Species |
Product Description |
Structure |
Purity |
Feature |
| TAT-K004 |
Human |
Anti TIGIT ELISA Kit |
|
|
|
| TIT-C52H7 |
Canine |
Canine TIGIT Protein,
His Tag |
|
 |
 |
| TIT-H5253 |
Human |
Human TIGIT Protein,
Mouse IgG2a Fc Tag, low endotoxin |
 |
 |
 |
| TIT-R5259 |
Rabbit |
Rabbit TIGIT Protein,
Fc Tag |
 |
 |
 |
| TIT-R5258 |
Rat |
Rat TIGIT Protein,
Fc Tag |
 |
 |
 |
| TIT-C5254 |
Cynomolgus / Rhesus macaque |
Cynomolgus / Rhesus macaque
TIGIT Protein, Fc Tag |
 |
 |
 |
| TIT-H82E5 |
Human |
Biotinylated Human TIGIT Protein, Avitag™,
His Tag (recommended for biopanning) |
 |
 |
 |
| TIT-C5223 |
Cynomolgus / Rhesus macaque |
Cynomolgus / Rhesus macaque TIGIT Protein,
His Tag (HPLC-verified) |
 |
  |
 |
| TIT-H82F1 |
Human |
Biotinylated Human TIGIT Protein, Fc,Avitag™ |
 |
 |
 |
| TIT-M52E6 |
Mouse |
Mouse TIGIT Protein,
His Tag |
 |
 |
 |
| TIT-M5257 |
Mouse |
Mouse TIGIT Protein,
Fc Tag |
 |
 |
 |
| TIT-H52H3 |
Human |
Human TIGIT Protein,
His Tag |
 |
 |
  |
| TIT-H5254 |
Human |
Human TIGIT Protein,
Fc Tag |
 |
 |
 |
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.