
Image Credit: ACROBiosystems
Antibody-Drug Conjugates (ADCs) are a fundamental technology in modern tumor-targeted therapy with their ‘tripartite integrated design’ of ‘precision carrier - stable linker - potent payload’:
- The carrier component comprises highly specific monoclonal antibodies, usually of the IgG1 isotype, which are meticulously screened to target tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs), such as HER2, TROP2, and CD22.
- The payload component consists of highly potent cytotoxic drugs. These drugs demonstrate elevated in vitro cytotoxicity but are too systemically toxic for standalone clinical application, so they require target ADC delivery to minimize risk.
- The linker component is a crucial ‘bridge’ between the antibody and the payload, requiring two essential properties:
First, high stability in the bloodstream (under neutral pH and low enzymatic activity conditions) is necessary to prevent premature payload detachment, which could result in free drug diffusion and off-target side effects such as myelosuppression or gastrointestinal toxicity.
Second, precise cleavage upon entry into tumor cells, triggered by the acidic environment of endosomes/lysosomes or elevated lysosomal enzyme activity, is needed to ensure efficient free payload release.
This design allows ADCs to overcome the limitations of traditional chemotherapy's "systemic killing" mode. After administering, the antibody carrier binds specifically to target antigens on the surface of tumor cells through its Fab region.
The resulting ‘ADC-antigen complex’ triggers the active uptake process of tumor cells, enabling precise delivery of the otherwise highly toxic cytotoxic payload into the cell interior.
This mechanism greatly decreases drug exposure to normal tissues. The entire process of ‘targeted binding - precise delivery - low toxicity and high efficacy’ is dependent on a central mechanism of ADC action, endocytosis.
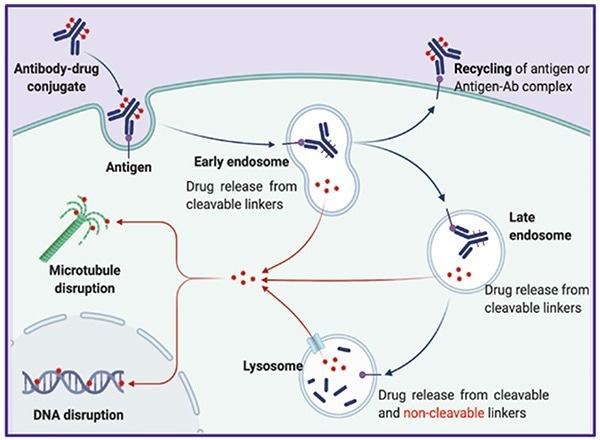
The Classic Mechanism of Action of ADCs. Image Credit: https://doi.org/10.1038/s41392-022-00947-7
Endocytosis detection is therefore both a crucial step in drug screening and mechanism optimization. Quantifying endocytosis efficiency enables accurate prediction of in vivo ADC behavior and provides essential data support for clinical evaluation.
Mainstream detection technologies for antibody internalization
Cell imaging technology
Principle -
To image cells, antibodies are first labelled with fluorescent dyes. After co-incubation with target cells, a fluorescence microscope is used to observe the localization change of fluorescent signals within the cells. When internalization occurs, fluorescence enters the cell interior, forming punctate or vesicular signals, which can be confirmed by co-localization with lysosomal markers.
| Advantages |
Disadvantages |
- High intuitivity: Directly visualizes the spatial distribution of fluorescent signals, clearly distinguishing antibodies bound to the cell membrane from those internalized into the cell.
- Enables co-localization: Can be combined with lysosomal and endosomal markers to clarify the transport pathway of antibodies following internalization.
|
- Qualitative-oriented: Unable to precisely quantify internalization efficiency, relying instead on subjective counting or semi-quantitative analysis.
- Signal interference susceptibility: Residual fluorescence from uninternalized antibodies remaining on the cell membrane surface may overlap with intracellular signals.
|
Flow cytometry (FCM)
Principle
Target cells are incubated with fluorescently labeled antibodies, followed by an acidic washing step to remove uninternalized antibodies from the cell membrane surface.
Flow cytometry is then employed to measure the intensity of intracellular fluorescent signals, which correlates positively with the number of antibodies internalized into the cells, thereby enabling quantitative analysis of internalization efficiency.
| Advantages |
Disadvantages |
- Accurate quantification: Provides direct quantification of intracellular fluorescence intensity, such as MFI value, allowing comparisons of internalization efficiency under varying conditions.
- Relatively high throughput: Compatible with 96-well plates, supporting batch analyses during antibody screening and process optimization.
|
- Absence of spatial information: Only detects the average fluorescence intensity of the entire cell population.
- False positives: Residual fluorescence on the membrane surface will cause false positives and underestimate internalization efficiency.
- Inability to track dynamically: Detection requires reaction termination using the end-point method, preventing the real-time observation of the internalization process.
|
Toxin Conjugate Assay
Principle
A Toxin Conjugate Assay is an indirect functional detection technique. Its underlying principle depends on the binding of primary antibodies (target antigen-specific antibodies) and secondary antibody-toxin conjugates (antibodies recognizing primary antibodies conjugated with cytotoxic toxins).
The internalization efficiency of primary antibodies is inferred indirectly based on the survival status of target cells. The antibody-toxin conjugates are co-transported into the cell only after successful internalization, where released toxins lead to cell death.
The cell survival rate is therefore negatively correlated with the internalization efficiency of primary antibodies.
| Advantages |
Disadvantages |
- High sensitivity: This technology exhibits significantly high sensitivity due to the signal amplification effect mediated by toxins. It is compatible with 96-well/384-well plates and supports high-throughput screening of antibody libraries.
|
- Non-specific effects: The technology is prone to non-specific cytotoxic effects and may be influenced by non-specific toxicity as it cannot distinguish between changes in internalization pathways (e.g., clathrin-dependent versus clathrin-independent).
|
ADC internalization detection antibody – labeled with pH-sensitive fluorescent dye
Given the critical importance of endocytosis detection in ADC research and development, ACROBiosystems has designed an antibody endocytosis detection reagent based on pH-sensitive dyes (Cat. No.: IGG-PZF2001) to meet the demand for endocytosis detection of ADC drugs.
This reagent employs a pH-sensitive fluorescent dye that labels Fab fragments. The dye then specifically binds to the Fc portion of the antibody under evaluation.
A stable antibody-dye complex can be formed within 10 minutes, providing a rapid assessment of the antibody internalization process. After labeling, the antibody can be detected using various techniques, including flow cytometry and cell imaging technology.
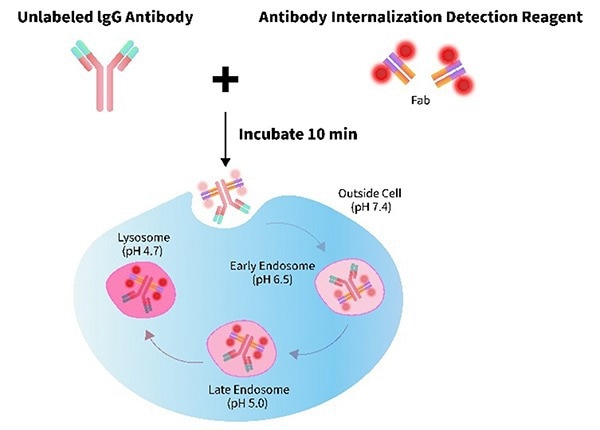
Image Credit: ACROBiosystems
Product features
- High signal-to-noise ratio: Strong fluorescence with minimal background
- Quick labeling: Complete in only 10 minutes
- pH-sensitive: Bright signal in acidic intracellular compartments
- Fab region preserved: Antibody binding activity remains unaffected.
Applications
- Discovery stage: Identification of antibodies that bind specifically to tumor-associated antigens and internalize efficiently
- Lead optimization: Evaluation of the effect of variations in antibody structure or conjugation chemistry on internalization and intracellular trafficking
- Preclinical development: Verification of consistent internalization behavior of the selected ADC candidate across relevant cell models
- Mechanism-of-action studies: Linking intracellular payload delivery and its correlation with cytotoxic effects.
Validation data
FACS analysis of antibody internalization
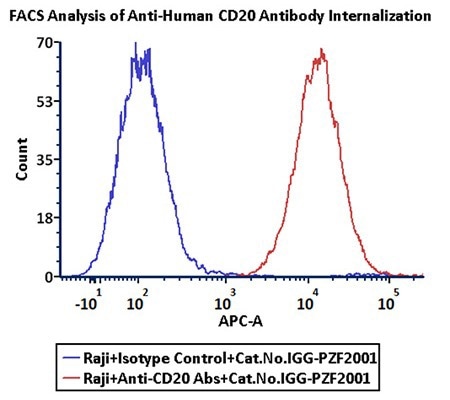
Anti-CD20 Abs and Human IgG1 isotype control were labeled with Antibody Internalization Detection Reagent (Cat.No.IGG-PZF2001). Raji cells were treated with Anti-CD20 Abs-Internalization Detection Reagent conjugate and Isotype control-Internalization Detection Reagent conjugate separately for two hours, then analyzed by Flow cytometry. APC signal was used to evaluate the activity (Routine tested). Image Credit: ACROBiosystems
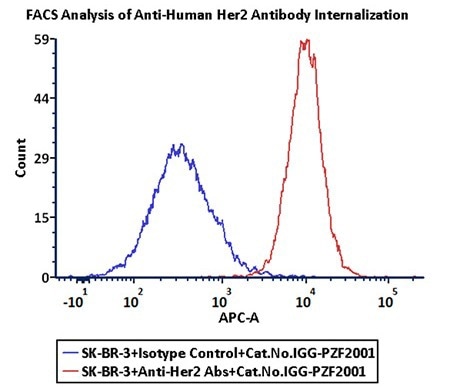
Anti-Her2 Abs and Human IgG1 isotype control were labeled with Antibody Internalization Detection Reagent (Cat.No.IGG-PZF2001). SK-BR-3 cells were treated with Anti-Her2 Abs-Internalization Detection Reagent conjugate and Isotype control-Internalization Detection Reagent conjugate separately for two hours, then analyzed by Flow cytometry. APC signal was used to evaluate the activity (Routine tested). Image Credit: ACROBiosystems
Fluorescence imaging of antibody internalization
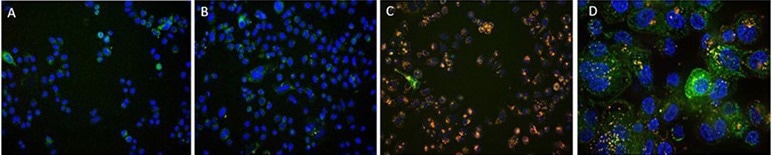
A. Antibody Internalization Detection Reagent (Cat.No.IGG-PZF2001). B. IgG1 Isotype+Internalization Detection Reagent conjugate. C. Anti-Her2 Abs+Internalization Detection Reagent conjugate. D. Anti-Her2 Abs+Internalization Detection Reagent conjugate (Z-stacking). (Green: CellLights Lysosome GFP, Blue: NucBlue Live ReadyProbes, Red: IGG-PZF2001, Cell line: SK-BR-3 Her2+). Image Credit: ACROBiosystems
The IGG-PZF2001 reagent accelerates the research and development of targeted anti-cancer therapeutics by enhancing the efficiency and accuracy of ADC endocytosis detection, providing a core driving force for the process.
References and further reading:
- Shivatare, V.S., et al. (2023). Probing the Internalization and Efficacy of Antibody‐Drug Conjugate via Site‐Specific Fc‐Glycan Labelling of a Homogeneous Antibody Targeting SSEA‐4 Bearing Tumors. Israel Journal of Chemistry, 63(10-11). https://doi.org/10.1002/ijch.202300042.
- Fu, Z., et al. (2022). Antibody drug conjugate: the ‘biological missile’ for targeted cancer therapy. Signal Transduction and Targeted Therapy, (online) 7(1). https://doi.org/10.1038/s41392-022-00947-7.
- ProBio. (2025). ADC Bioassay Service | Antibody Internalization Assay - ProBio CDMO. (online) Available at: https://www.probiocdmo.com/add-adc-bioassay-service.html.
- Nath, N., et al. (2016). Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye. Journal of Immunological Methods, 431, pp.11–21. https://doi.org/10.1016/j.jim.2016.02.001.
- Li, Y., et al. (2015). A Cell-Based Internalization and Degradation Assay with an Activatable Fluorescence-Quencher Probe as a Tool for Functional Antibody Screening. Journal of biomolecular screening, (online) 20(7), pp.869–75. https://doi.org/10.1177/1087057115588511. .
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.