
Image Credit: ACROBiosystems
September 21, 2025, marked the 32nd World Alzheimer’s Day. Alzheimer’s disease (AD) is the primary cause of dementia. Often linked to aging, it is a progressive neurodegenerative disorder, not a natural part of getting older.
This disorder causes neuronal damage in the brain, progressively impairing memory and cognitive function. In its advanced stages, it disrupts basic physiological functions.
In recent years, progress has been made in comprehending the disease’s biological mechanisms, enhancing early diagnosis, and advancing therapeutic development. Nevertheless, existing treatments provide only symptomatic relief or modestly delay disease progression, and cannot fundamentally reverse its course. The development of more effective and targeted therapies is an urgent priority.
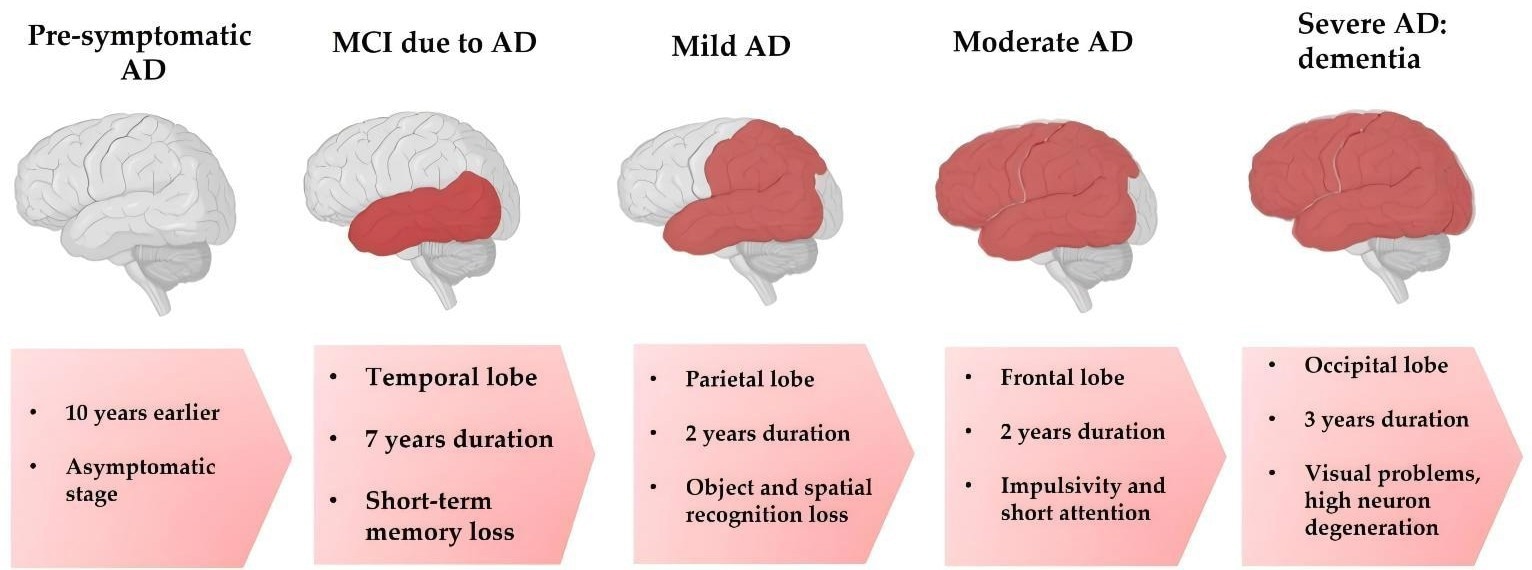
Progressive stages in the development of AD. Image Credit: https://doi.org/10.3390/nu15173688
The pathological network of AD: Core and synergistic mechanisms
There are two interacting cascades at the centre of Alzheimer’s pathology: abnormal Aβ deposition and tau hyperphosphorylation.
Amyloid precursor protein (APP) is sequentially cleaved by β-secretase (BACE1) and γ-secretase, producing Aβ peptides that assemble into neurotoxic oligomers. Through receptors such as Nogo, these oligomers bind to neuronal membranes, resulting in calcium imbalance, mitochondrial dysfunction, and impaired synaptic transmission.
At the same time, dysregulated kinases such as GSK-3β, CDK5, and MAPKs, drive tau hyperphosphorylation, causing tau to detach from microtubules and aggregate into neurofibrillary tangles. This disruption of axonal transport leads to neuronal loss and progressive cognitive deterioration.
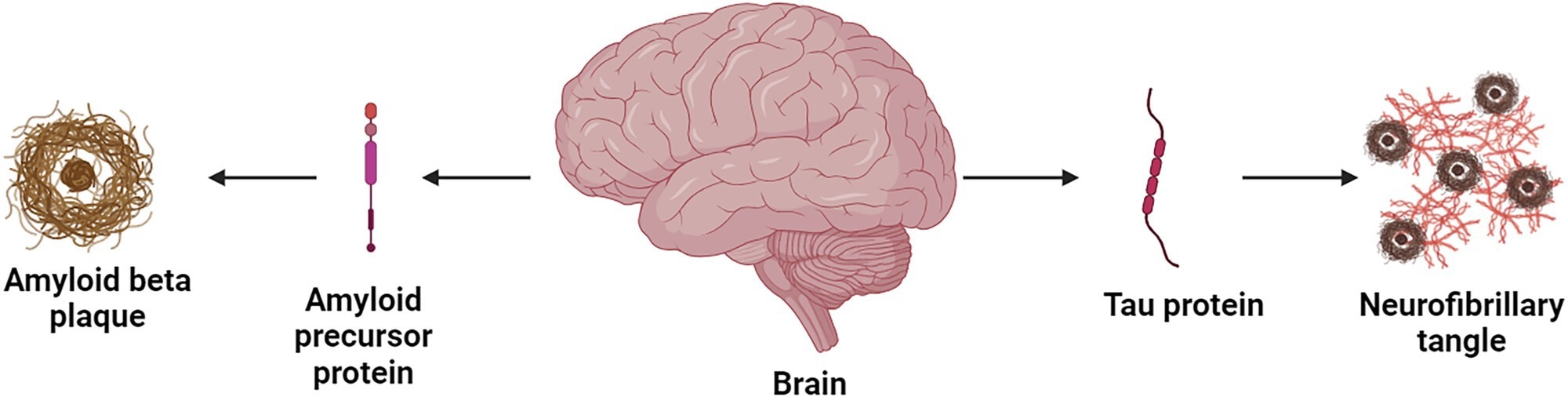
The degenerative cascade in AD. Image Credit: https://doi.org/10.1002/ibra.12197
In addition to these primary characteristics, a variety of synergistic mechanisms contribute to AD:
Cholinergic impairments, where AChE and BChE activity reduces acetylcholine insufficiency; neuroinflammation, linked to risk factors such as the APOE4 allele and TREM2-mediated microglial dysfunction, which compromises Aβ clearance; synaptic dysfunction, including NPTX2 downregulation and Nogo receptor-mediated inhibition of neuronal plasticity; and metabolic abnormalities, such as progranulin deficiency and lipid metabolism disorders.
Alongside Aβ and tau, these mechanisms form an intricate pathological network that propels the progression of AD.
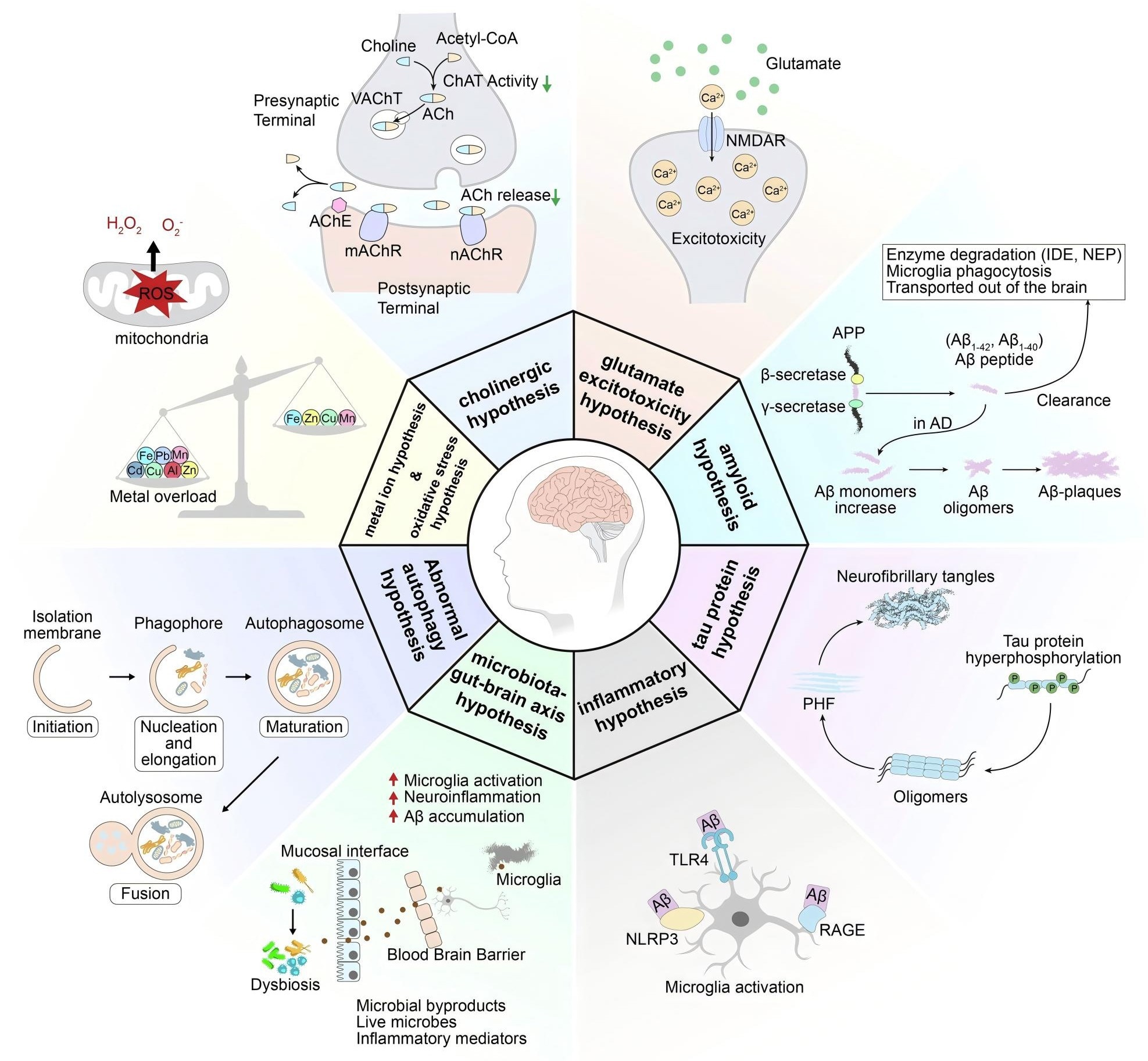
Diagram for the pathogenesis of AD. https://doi.org/10.1038/s41392-024-01911-3
Therapeutic development: From symptomatic relief to disease modification
-
Conventional symptomatic treatment: Addressing neurotransmitter deficits
For many years, clinical management of AD has relied primarily on cholinesterase inhibitors, including donepezil, rivastigmine, and galantamine, and NMDA receptor antagonists like memantine.
These drugs function by increasing acetylcholine levels or modulating glutamatergic signaling in the brain. While they partially improve cognitive symptoms and behavioral abnormalities, they are unable to stop the underlying neurodegenerative process.
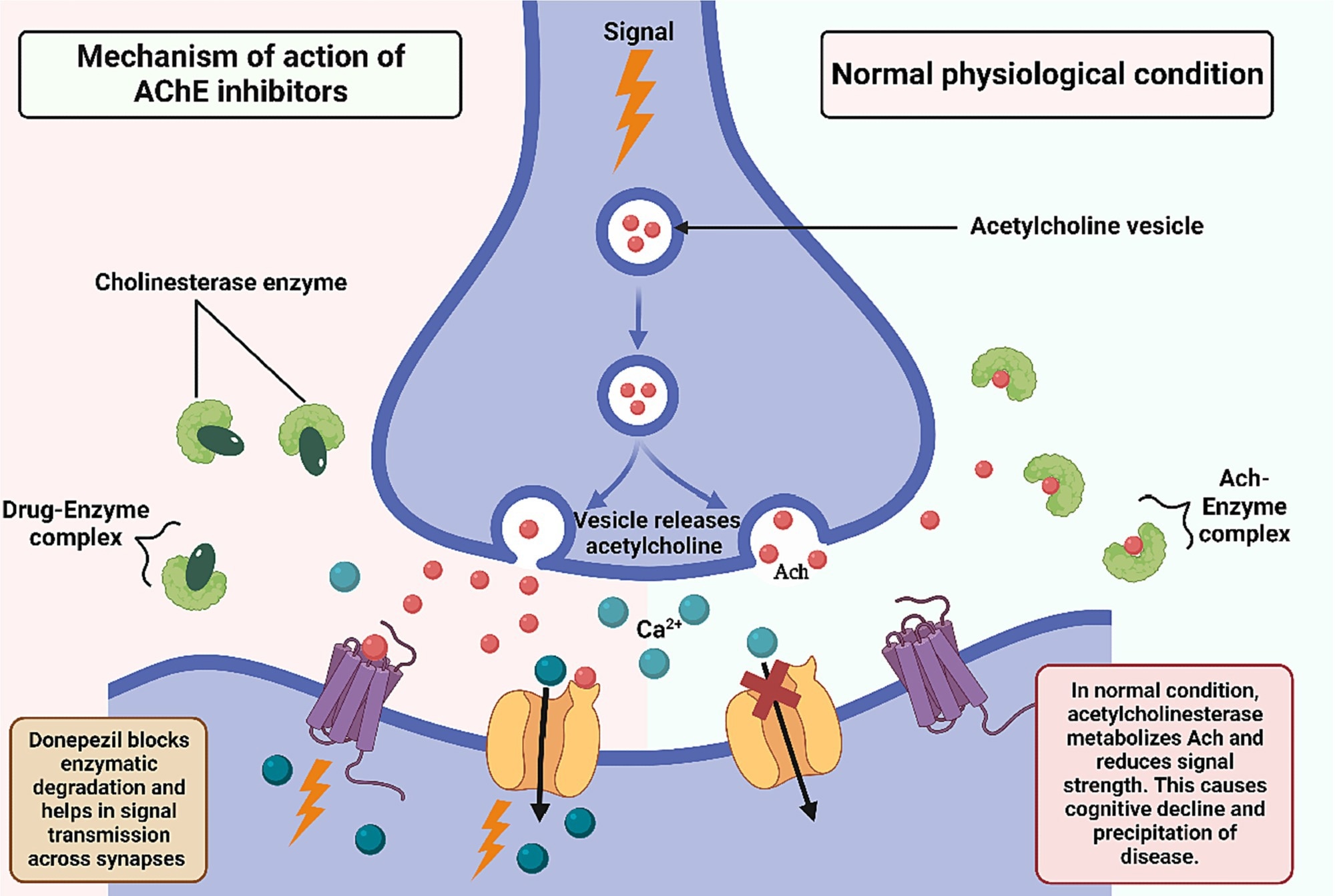
Mechanism of action of cholinesterase inhibitors. Image Credit: https://doi.org/10.1016/j.jconrel.2024.01.047
-
Targeting Aβ for disease modification: Promise and challenges
Immunotherapies targeting Aβ represent a significant advancement in recent AD therapies. Monoclonal antibodies such as lecanemab and donanemab are now approved for early-stage AD treatment, having shown success in effectively reducing cerebral Aβ plaques and substantial efficacy in delaying cognitive deterioration in clinical trials.
However, limitations persist, including potential side effects such as amyloid-related imaging abnormalities (ARIA), and benefits remain limited in moderate-to-advanced disease stages.
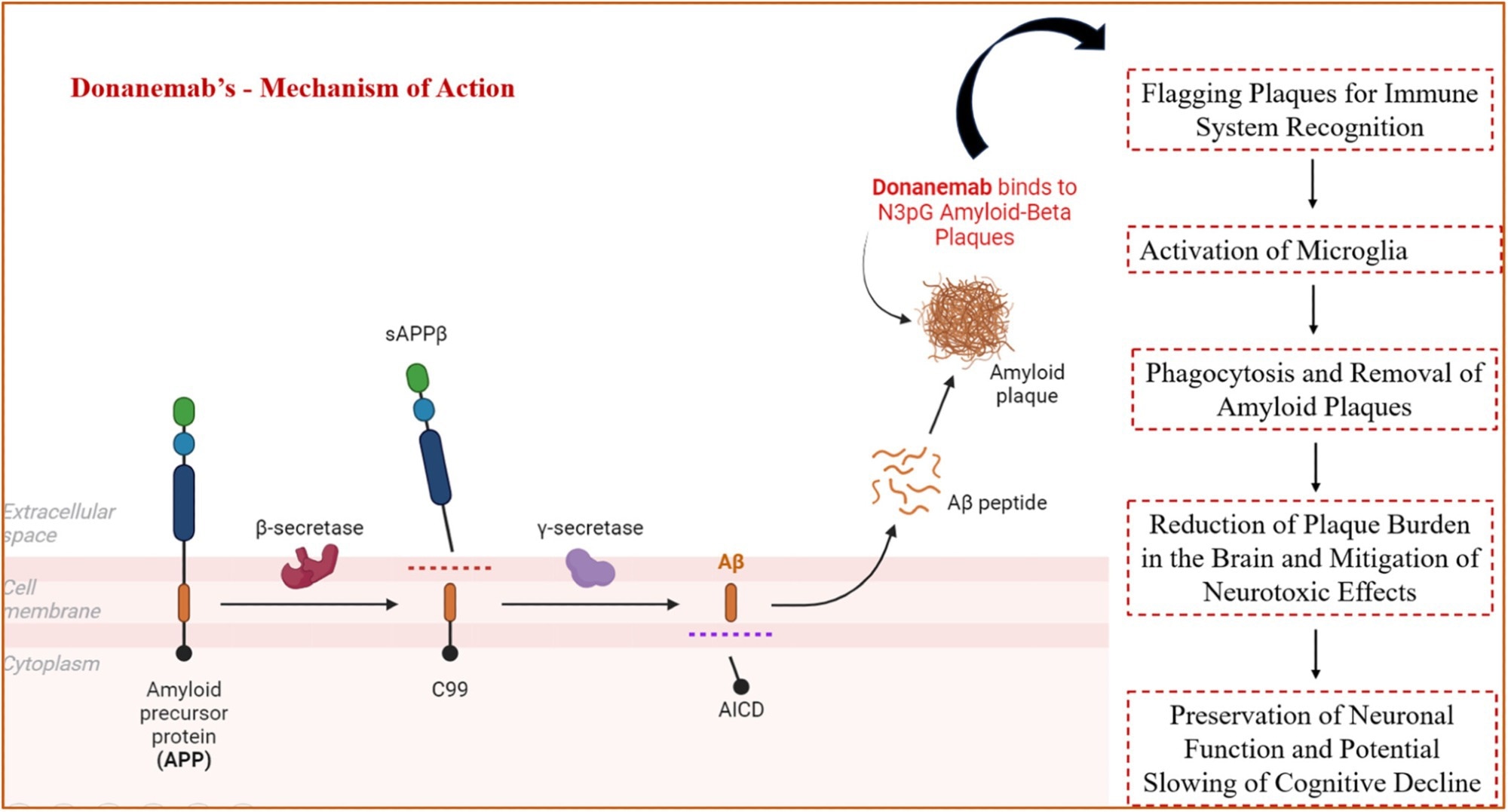
Mechanism of action of donanemab. Image Credit: https://doi.org/10.1002/cdt3.155
-
Investigating novel targets: Multi-target approaches for the future
As understanding of AD pathogenesis develops, therapeutic development is moving beyond the conventional Aβ-targeting strategy, progressing toward multi-target and multi-pathway techniques:
Anti-tau treatments: Including aggregation inhibitors, monoclonal antibodies, and vaccines developed to stop the formation and spread of neurofibrillary tangles.
Anti-neuroinflammation strategies: Targeting receptors such as TREM2 to modulate microglia from pro-inflammatory to protective phenotypes, thereby enhancing the removal of pathological proteins.
Metabolic and neurotrophic modulation: Enhancing brain energy metabolism and promoting neuronal survival through mechanisms such as improved brain insulin signaling.
Multi-target agents: Developing small molecules that act on multiple pathways concurrently to achieve synergistic therapeutic effects. For example, combining cholinesterase inhibition with modulation of GSK-3β or MAO-B.
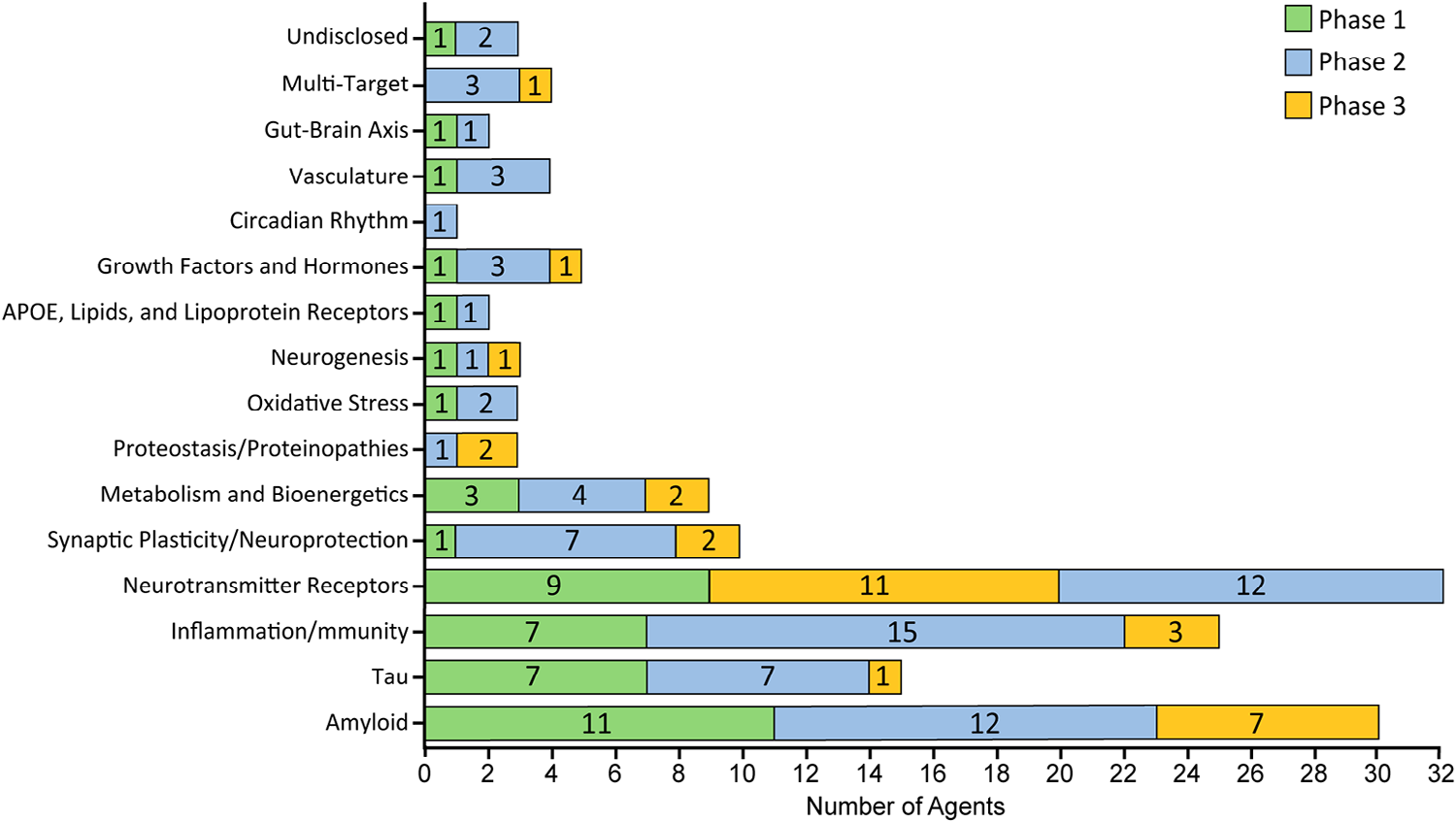
Number of AD drug candidates by mechanism of action across different stages of development. Image Credit: https://doi.org/10.1002/trc2.70098
-
ACROBiosystems’ solutions: Driving progress in AD research
ACROBiosystems’ neuroscience brand, Aneuro, accelerates AD research and therapeutic development by offering innovative solutions, including target proteins, pre-formed fibrils (PFFs), stable cell lines, and p-tau antibodies.

Image Credit: ACROBiosystems
References and further reading:
- Pinto-Hernandez, P., et al. (2023). Modulation of microRNAs through Lifestyle Changes in Alzheimer’s Disease. Nutrients, 15(17), pp.3688–3688. https://doi.org/10.3390/nu15173688.
- Mohapatra, T.K., et al. (2025). Navigating the treatment landscape of Alzheimer’s disease: Current strategies and future directions. Ibrain, 11(2), pp.162–184. https://doi.org/10.1002/ibra.12197.
- Zhang, J., et al. (2024). Recent Advances in Alzheimer’s disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduction and Targeted Therapy, [online] 9(1). https://doi.org/10.1038/s41392-024-01911-3.
- Singh, B., et al. (2024). Alzheimer’s disease current therapies, novel drug delivery systems and future directions for better disease management. Journal of Controlled Release, 367, pp.402–424. https://doi.org/10.1016/j.jconrel.2024.01.047.
- Nandhini Jayaprakash and Karthikeyan Elumalai (2024). Translational Medicine in Alzheimer’s Disease: The Journey of Donanemab From Discovery to Clinical Application. Chronic Diseases and Translational Medicine. https://doi.org/10.1002/cdt3.155.
- Cummings, J.L., et al. (2025). Alzheimer’s disease drug development pipeline: 2025. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 11(2). https://doi.org/10.1002/trc2.70098.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.