The first Saturday of May each year marks World Ankylosing Spondylitis (AS) Day, raising awareness of the 54 million patients globally living with spinal rigidity, morning stiffness and pain, bone spur proliferation, and even irreversible disability.
Statistics show that without standardized treatment, 65% of AS patients will develop disabilities within 10 years. However, early diagnosis and treatment can control 80% of disease progression.
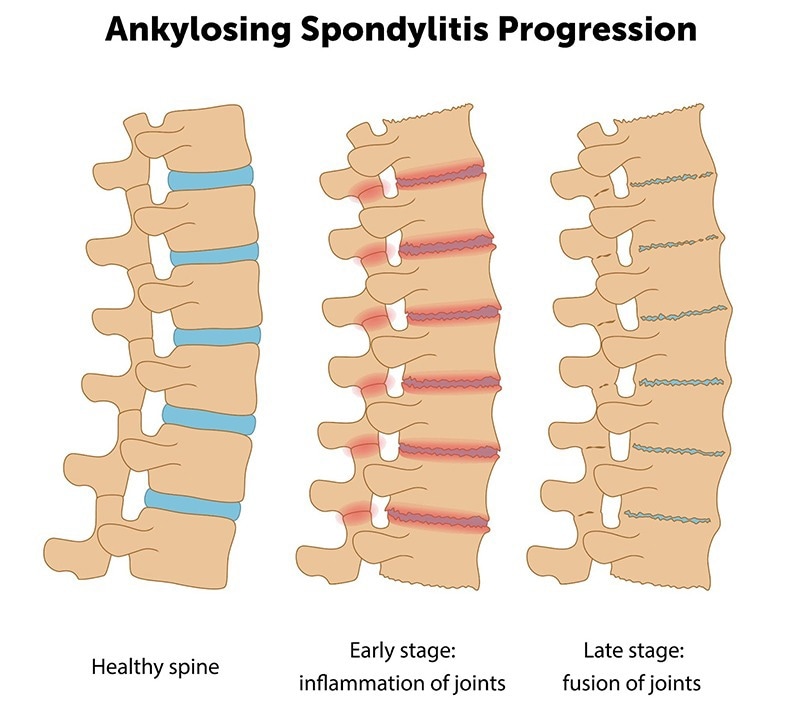
Progression of AS. Image Credit: ACROBiosystems
AS is a chronic inflammatory autoimmune disorder principally characterized by axial spine and sacroiliac joint inflammation. Patients may develop severe complications as the disease progresses, such as restricted thoracic mobility, spinal fusion (manifesting as ‘bamboo spine’ structural changes), and hip joint destruction.
The introduction of biologic therapies has transformed AS management, but a subset of patients still faces drug tolerance challenges or demonstrate suboptimal responses to current treatments.
This unmet medical need has driven research institutions and pharmaceutical companies around the world to prioritize the development of precision medicine approaches and novel targeted therapies able to address specific pathogenic mechanisms.
ACROBiosystems offers a comprehensive product portfolio for research into AS, including stable cell lines, highly active recombinant proteins, and inhibitor screening kits.
The company’s product offering spans the entire drug development continuum, including target discovery and validation, candidate drug screening and development, and CMC manufacturing and quality control. These tools are ideally suited to supporting the rapid and efficient translation of AS innovative therapies from foundational research to clinical implementation.
Decoding AS pathogenesis: HLA-B27 gene fuels TNF-α/IL-17 bone destruction cascade
AS develops via a multifaceted interplay of immune, infectious, genetic, and environmental factors. While the HLA-B27 gene is not the sole determinant, it does function as a molecular ‘trigger,’ priming carriers’ immune systems to hyperreact to specific antigens.
The immune system mistakenly identifies HLA-B27-associated joint tissues as foreign threats when pathogens such as Klebsiella invade the gut, unleashing a storm of inflammatory cytokines such as interleukin-17 (IL-17) and tumor necrosis factor-alpha (TNF-α)).
This immune dysregulation triggers a domino effect: TNF-α accelerates bone resorption by hyperactivating osteoclasts; IL-17 narrows joint spaces by driving fibroblast proliferation; and inflammatory cascades are amplified by an imbalance in Th17/Treg cell ratios.
These mechanisms collectively shift the disease trajectory from transient inflammatory pain to irreversible structural bone damage, ultimately resulting in spinal fusion and rigidity.
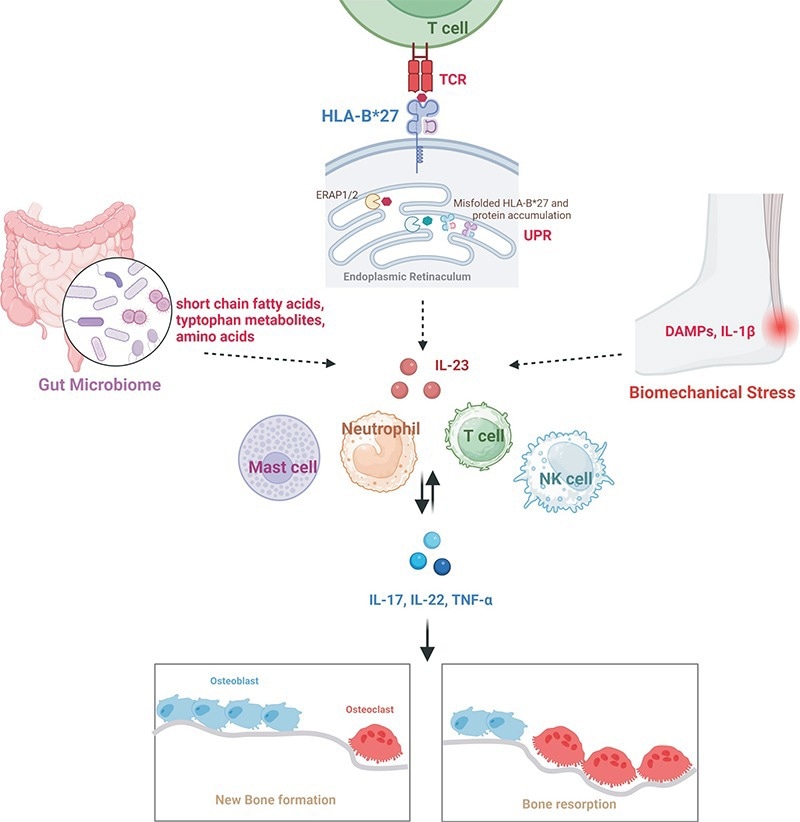
AS and its pathogenic mechanisms. Image Credit: ACROBiosystems
Dual-engine strategy revolutionizes AS treatment: Precision targeting meets regenerative medicine
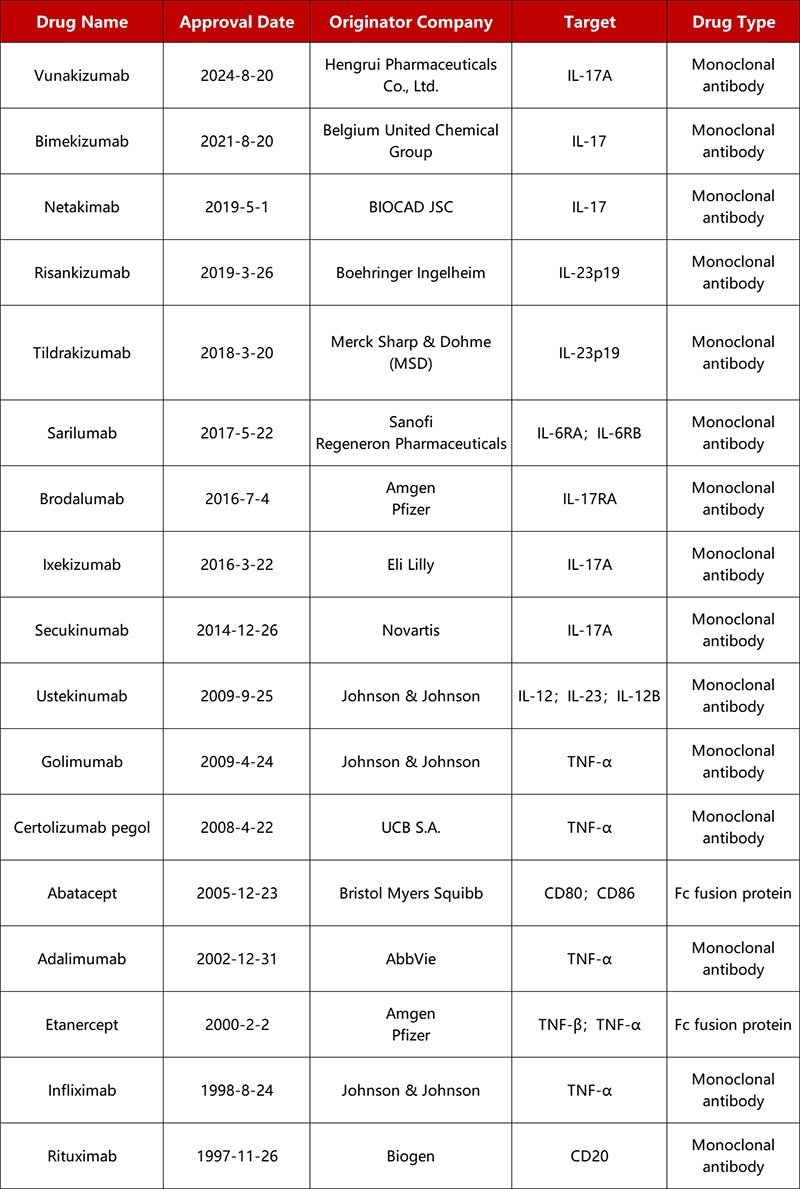
Approved therapies for AS typically function by targeting key molecules in inflammatory pathways, including IL-17, IL-23, and TNF-α.
The earliest approved biologics include TNF-α inhibitors such as adalimumab and infliximab. These biologics alleviate joint inflammation by blocking the pro-inflammatory cytokine TNF-α.
Adalimumab (Humira) historically dominated the market as the global ‘blockbuster drug’ with annual sales exceeding $20 billion, but following patent expiration in 2023, its position has suffered due to biosimilars entering the market.
IL-17/IL-23 pathway inhibitors, targeting upstream inflammatory mediators, have become widely regarded as mainstream options due to their superior clinical response rates.
For example, Novartis' secukinumab (IL-17A inhibitor) achieved $4.788 billion in sales in 2022, while Eli Lilly's ixekizumab reached $2.894 billion. UCB's bimekizumab also rapidly gained market traction due to its unique dual-targeting mechanism able to target IL-17A/F.
IL-23 inhibitors such as tildrakizumab and risankizumab exhibit improved safety profiles via precise p19 subunit inhibition, with risankizumab reaching more than $7.9 billion in global sales by 2023.
China's first domestically developed IL-17A inhibitor, Hengrui Pharmaceuticals' self-developed fully humanized frexizumab was approved in 2024. This emerging therapy reduces immunogenicity, marking a breakthrough for domestic pharmaceutical companies in the AS field.
Approved originator biologics for AS. Source: Pharmacodia
Groundbreaking new research is exploring regenerative therapeutic strategies that can surpass traditional immunosuppression. For instance, drug development leveraging the gut-joint axis theory represents a disruptive new frontier, with oral sphingosine-1-phosphate receptor modulators demonstrating dual mechanisms of action and offering the ability to simultaneously regulate lymphocyte migration and restore intestinal barrier function. Early-phase clinical data suggest substantial reductions in C-reactive protein levels.
In cellular therapy, Treg cell expansion technologies and CAR-Treg therapies are being developed with the goal of re-establishing immune equilibrium. CRISPR-Cas9 gene-editing tools targeting HLA-B27 conformational abnormalities have also been found to successfully rectify antigen-presentation defects in preclinical models.
These pioneering advances drive the evolution of AS treatment paradigms from symptomatic management to disease-modifying interventions.
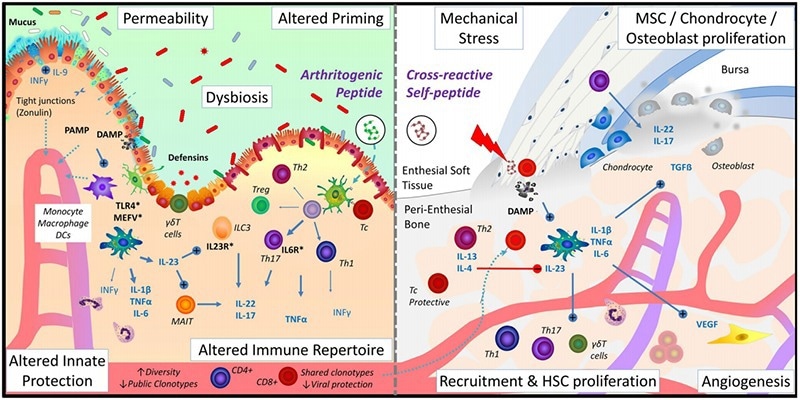
Immune regulation of the joint-gut-microbiome axis in AS. Image Credit: ACROBiosystems
ACROBiosystems pioneers AS therapeutics
AS is transitioning from a chronic, debilitating disease to a manageable and treatable chronic condition as understanding of AS heterogeneity improves and technological innovations accelerate. This work will be key to delivering transformative improvements in quality of life for patients worldwide.
ACROBiosystems has developed a comprehensive product portfolio designed to support this work, including stable cell lines, highly active recombinant proteins, and inhibitor screening kits.
The company’s solutions span the complete drug development continuum, ranging from target discovery and validation to candidate drug screening and development, and CMC manufacturing and quality control.
ACROBiosystems’ product offering is designed to support and expedite the efficient translation of AS innovative therapies from foundational research to clinical implementation.
Hot AS Target Recommendations. Source: ACROBiosystems
| . |
. |
. |
. |
. |
| TNF-alpha |
IL-17A |
TNF-beta |
IL-17A & IL-17F |
IL-12/IL-23 p40 |
| CD20 |
IL-17F |
IL-12B |
IL-17 RA |
IL-6 R alpha |
| IL-6 R beta |
PD-1 |
TNFR2 |
IL-23A |
GM-CSF |
ComboX, a combination of universal solutions

References and further reading
- Se Hee Kim and Lee, S.-H. (2023). Updates on ankylosing spondylitis: pathogenesis and therapeutic agents. Journal of rheumatic diseases, 30(4), pp.220–233. https://doi.org/10.4078/jrd.2023.0041.
- Garrido-Mesa, J. and Brown, M.A. (2022). T cell Repertoire Profiling and the Mechanism by which HLA-B27 Causes Ankylosing Spondylitis. Current Rheumatology Reports, (online) 24(12), pp.398–410. https://doi.org/10.1007/s11926-022-01090-6.
- Perrotta, F.M., et al. (2022). Therapeutic Targets for Ankylosing Spondylitis – Recent Insights and Future Prospects. Open Access Rheumatology: Research and Reviews, (online) 14, pp.57–66. https://doi.org/10.2147/OARRR.S295033.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.