Mycoplasma is a type of bacteria that is widely recognized as being one of the smallest organisms capable of independent survival. More than 190 species of mycoplasma exist in humans, plants, and animals.
Unlike the majority of bacteria, mycoplasma lacks a cell wall. Instead, it survives by acquiring nutrients directly from host cells. Its minute size of just 0.15-0.3 microns allows it to evade filtration systems, meaning that it is hard to detect mycoplasma, even when present in high concentrations.
Mycoplasma contamination poses several risks to cell physiology, including interfering with DNA and RNA synthesis, inducing chromosomal abnormalities, altering membrane antigenicity, reducing transfection rates, inhibiting cell proliferation and metabolism, altering gene expression profiles, and triggering cell death.
Controlling mycoplasma contamination
A range of cell lines is employed in the production of therapeutic biologics such as vaccines, monoclonal antibodies, cytokines, and immunomodulators.
Advanced therapy medicinal products (ATMPs) based on gene and cell therapies have become increasingly popular as novel treatment options, but autologous cell therapy’s production involves a complex multi-stage process, with each stage presenting potential mycoplasma contamination risks.
Mycoplasma contamination is a substantial risk throughout the cell therapy and biopharmaceutical industries. A contaminated cell matrix may threaten patient safety, triggering product issues or recalls and resulting in serious economic losses.
Routine mycoplasma testing is, therefore, indispensable in biopharmaceutical research, development, and production.
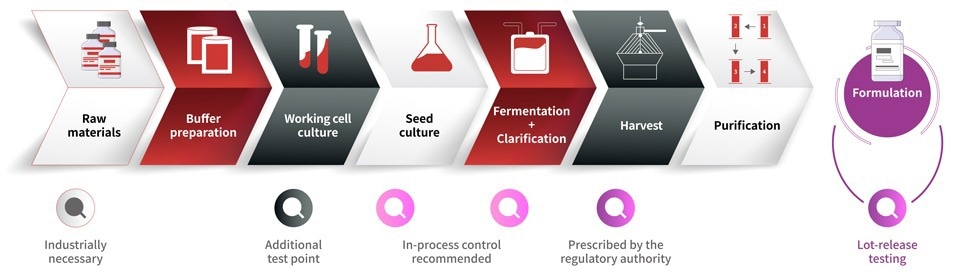
Monitoring Mycoplasma Contamination in Biopharmaceutical Production. Image Credit: Source: ACROBiosystems
Mycoplasma detection methods
Mycoplasma detection is a key consideration throughout biologics production using cell lines.
Common detection methods include DNA staining assays, indicator cell-based assays, culture-based assays, and PCR-based assays. Conventional culture-based and indicator cell-based assays are time-consuming (taking up to 28 days), cumbersome, and only offer low sensitivity.
Developments in cell-based therapies and ATMPs have resulted in increasingly stringent requirements around mycoplasma detection timeliness and sensitivity. PCR-based assays leveraging nucleic acid amplification technology (NAT) have emerged as a fast and dependable alternative, offering rapid setup and analysis, high specificity, sensitivity, reliability, and cost-effectiveness.
Major pharmacopeias like EP<2.6.7>, JP, and USP<63> all currently feature NAT methods for mycoplasma detection.
Rapid mycoplasma detection solutions
ACROBiosystems offers a comprehensive mycoplasma monitoring solution designed to accelerate in-process and batch release testing, featuring a mycoplasma DNA sample preparation kit and a mycoplasma rapid detection kit.
These kits enable reliable, sensitive mycoplasma contamination detection in biologics while adhering to the regulatory guidelines of key pharmacopeias (10 CFU/ml).
Image Credit: Source: ACROBiosystems
This mycoplasma monitoring solution offers a number of key advantages:
- Wide coverage, with the ability to detect more than 250 mycoplasma and spiroplasma species, including all specificities listed in EP, USP, and JP
- High specificity, with primers and probes designed to target mycoplasma 16S rRNA, therefore avoiding cross-reactivity with non-mycoplasma genera
- High sensitivity, confidently meeting or exceeding regulatory standards of 10 CFU/mL
- Strong compatibility, including suitability for complex matrices such as 10% DMSO and high cell density samples
- User-friendly, featuring a single-well assay design with included controls
- Rapid, with a total assay time of just 2.5 - 3 hours
- High quality, with all kits manufactured in GMP-like facilities
- Compliance, meeting ISO 13485 standards and validated according to relevant guidelines
- Comparable results, with assay results consistently comparable to culture-based methods
Source: ACROBiosystems
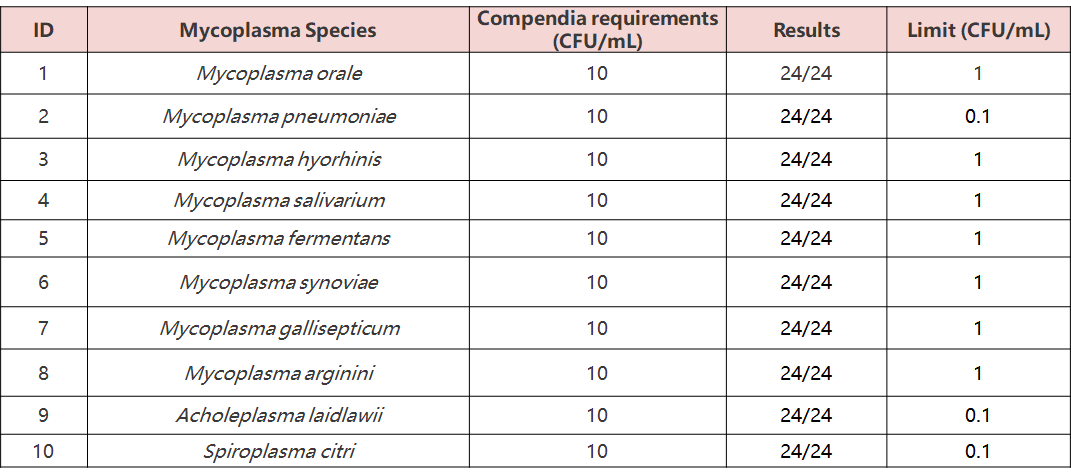
A total of 24 tests were conducted on 10 mycoplasma standards (10 CFU/mL), with all results found to be positive. These findings confidently meet or exceed the requirements of EP 2.6.7 (10 CFU/mL).
Source: ACROBiosystems
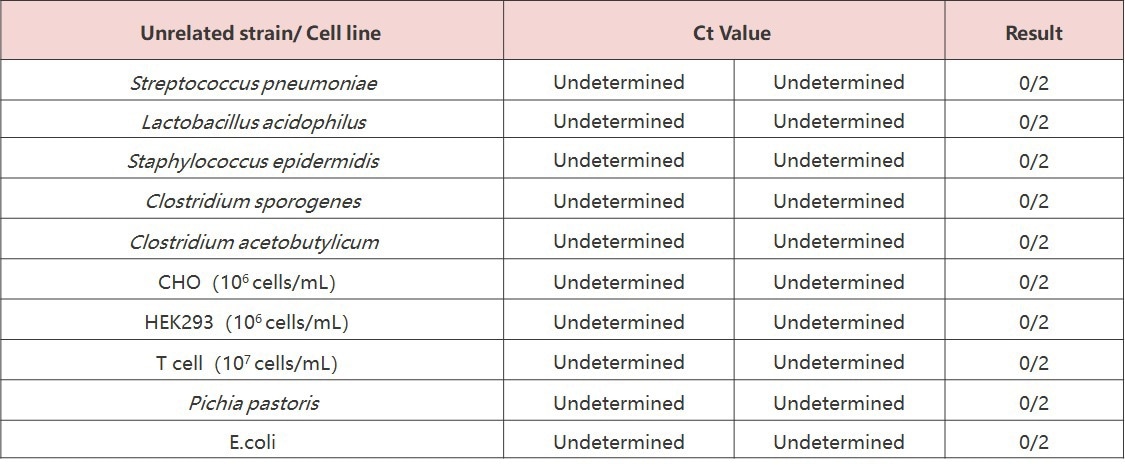
No cross-reactivity was observed when testing on two common cell lines and four unrelated bacterial strains (Streptococcus pneumoniae, Lactobacillus acidophilus, Staphylococcus epidermidis, and Bacillus subtilis). This confirmed that the test is not influenced by unrelated cells or strains.
Source: ACROBiosystems
References and further reading
- ATCC. Mycoplasma quality control of cell substrates and biopharmaceuticals. (online) Available at: https://www.atcc.org/resources/white-papers/mycoplasma-quality-control-of-cell-substrates-and-biopharmaceuticals.
- Young, L., et al. (2010). Detection of Mycoplasma in Cell Cultures. Nature Protocols, 5(5), pp.929–934. https://doi.org/10.1038/nprot.2010.43.
- Angart, P., et al. (2018). Considerations for risk and control of mycoplasma in bioprocessing. Current Opinion in Chemical Engineering, 22, pp.161–166. https://doi.org/10.1016/j.coche.2018.09.012.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.