Ensuring the safety and quality of pharmaceuticals, such as blood products, vaccines, recombinant proteins, and monoclonal antibodies, is essential. Endotoxins act as a hidden ‘time bomb’ in these pharmaceuticals, threatening product safety and indicating production flaws.
Among various detection methods for endotoxins, recombinant Factor C (rFC) testing stands out for its accuracy and eco-friendliness, establishing a new benchmark in pharmaceutical quality control.
Limitations of traditional endotoxin detection methods
Historically, the pharmaceutical industry relied on the Limulus Amebocyte Lysate (LAL) assay and the Rabbit Pyrogen Test for detecting endotoxins. Neither method is perfect.
Whilst the LAL assay is effective, it depends on horseshoe crabs, impacting ecological balance and exhibiting batch-to-batch variations. While it has less variation, the Rabbit Pyrogen Test is both costly and time-consuming and has low sensitivity that can lead to reduced detection of endotoxins.
Principles and advantages of recombinant Factor C endotoxin detection technology
Principle:
Recombinant Factor C endotoxin detection technology operates on the principle of activating Factor C in the horseshoe crab blood coagulation cascade by endotoxins.
Synthesized through genetic engineering, when recombinant Factor C interacts with endotoxins in a sample, it binds to specific targets and triggers a series of enzymatic reactions.
Fluorescence is used to measure the final endotoxin concentration, allowing for highly specific detection without interference from other substances.
Advantages:
High sensitivity
The recombinant Factor C detection method identifies extremely low levels of endotoxins, achieving sensitivity as low as 0.005 EU/mL. Much more sensitive than traditional techniques, this enables the detection of endotoxin contamination earlier in pharmaceutical processes, enabling timely corrective actions.
High specificity
Recombinant Factor C reacts specifically to endotoxins and is unaffected by other substances.
This results in more accurate and reliable detection, particularly important in complex sample compositions where traditional methods may yield false results.
Good stability
Unlike the LAL assay, recombinant Factor C is produced through genetic engineering, ensuring strict quality control and minimal variation between batches. This results in consistent and reliable outcomes over time.
Environmentally sustainable
rFC testing does not require rare biological resources like horseshoe crabs, and so avoids causing ecological harm. With a growing focus on green practices in the pharmaceutical sector, recombinant Factor C endotoxin detection shows strong potential.
Applications of recombinant Factor C endotoxin detection technology in pharmaceutical processes
Raw material detection
In pharmaceutical manufacturing, raw material quality directly influences the final product’s safety and efficacy. Endotoxin contamination in raw materials, particularly for blood products and vaccines, can accumulate during production and severely impact quality.
When using recombinant Factor C technology during sourcing, each batch can be rigorously tested to meet endotoxin limits, ensuring safety and quality. For example, endotoxin testing on collected plasma can prevent contamination in plasma product manufacturing.
Production process monitoring
Each phase of pharmaceutical manufacturing poses a risk of endotoxin contamination, making continuous monitoring of the production process vital.
By regularly testing production equipment, pipelines, and intermediates with recombinant Factor C technology, manufacturers can swiftly identify contamination sources and implement necessary cleaning procedures.
For example, in vaccine production, on-site testing of fermentation tanks can quickly halt operations if endotoxin levels exceed safe thresholds, preventing further contamination.
Finished product quality control
Testing finished products is the final safeguard to guarantee drug safety and effectiveness. After production, each batch must undergo thorough quality testing, including endotoxin assessment. There have been cases where bacterial endotoxin levels in medical devices surpassed safe limits, resulting in adverse reactions and recalls.
Research indicates that a significant percentage of FDA recalls were due to bacterial endotoxins in medical devices, underscoring the need for robust testing.
Recombinant Factor C technology provides rapid and accurate endotoxin measurement in final products, ensuring compliance with quality standards and protecting patient safety.
Conclusion
Recombinant Factor C endotoxin detection technology is crucial in pharmaceutical manufacturing for enhancing product safety and supporting sustainability goals.
Its adoption is endorsed by major pharmacopoeias, including the U.S. Pharmacopeia (2024) and the European Pharmacopoeia (2023), which recognize rFC as a standard for bacterial endotoxin testing.
ACROBiosystems recombinant Factor C endotoxin detection kit
The Recombinant Factor C Endotoxin Detection Kit (Cat. NO. RES-A056) from ACROBiosystems offers a rapid and effective method to detect bacterial endotoxins in samples.
The endotoxin standards in the kit strictly align with USP standards (Cat. No. U1235503) and China’s bacterial endotoxin working standards (No. 150601).
This kit is designed to meet the stringent quality control requirements of the pharmaceutical, medical device, and biopharmaceutical industries.
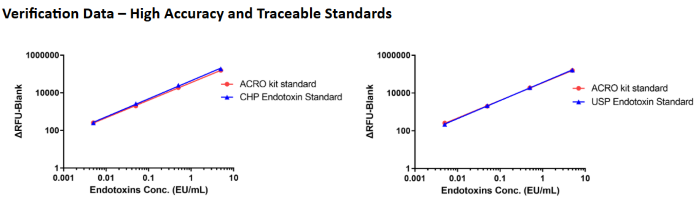
Using endotoxin (CHP, Cat. No. 150601 & USP, Cat. No. U1235503) as the standard, the recovery rate of the ACRO Kit standard falls within the 50%-200% range, meeting regulatory requirements. Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.