Biomarkers are key to the development of biological drugs, offering vital insights into drug mechanisms of action (MOA), informing clinical study design, and optimizing treatment strategies.
Biomarkers see widespread use across early discovery, preclinical, and clinical stages, accelerating drug development by ensuring precise dosing and efficacy evaluation.
This article outlines the MOA and biomarker research for a number of biological drugs developed for the treatment of autoimmune diseases.
Mechanisms of action of biological drugs in autoimmune disease
A significant amount of biological drug research is focused on the treatment of autoimmune diseases, due to their well-characterized mechanisms and specific targets.
The leading indications under development include Crohn’s disease, plaque psoriasis, ankylosing spondylitis (AS), rheumatoid arthritis, arthritis, and ulcerative colitis, with these conditions collectively accounting for more than 1,100 drug candidates currently in development pipelines.
Primary therapeutic targets in this field encompass the TNF/IL-23/IL-17 axis, with critical biomarkers and cytokines including TNF-α, IL-23p19, IL-17A, IL-12/23p40, IL-17F, and TL1A.
Several targeted drugs already are on the market, most notably adalimumab, ustekinumab, secukinumab, guselkumab, tildrakizumab, and risankizumab (Figure 1).
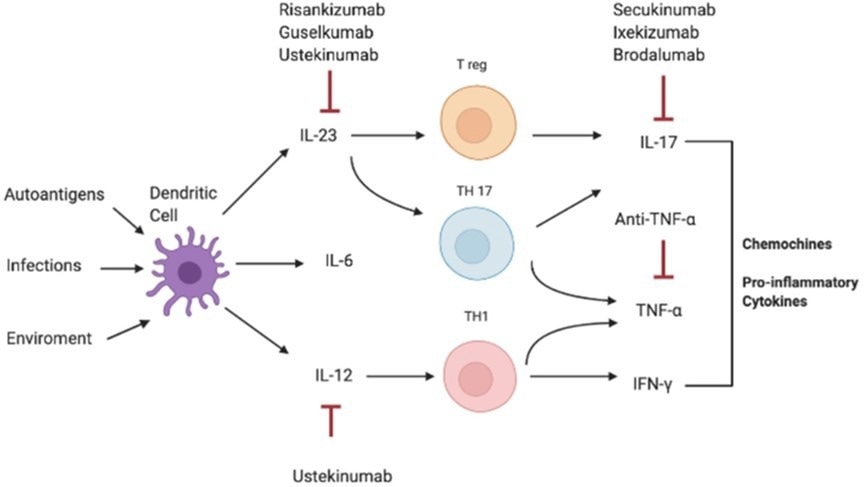
Figure 1. Pathogenesis of IL-17-correlated disease and different targets of therapy. Image Credit: https://doi.org/10.3389/fimmu.2021.637829.
The type 2 inflammation response represents another key pathway. This pathway is primarily mediated by type 2 innate lymphoid cells, Th2 cells, and Th2 cytokines such as IL-4, IL-5, IL-13, and IL-31.
Relevant biomarkers include cytokines and targets such as IL-4, IgE, IL-5, IL-13, IL-33, and TSLP (Figure 2). A number of targeted drugs are currently available, including lebrikizumab, dupilumab, and mepolizumab.
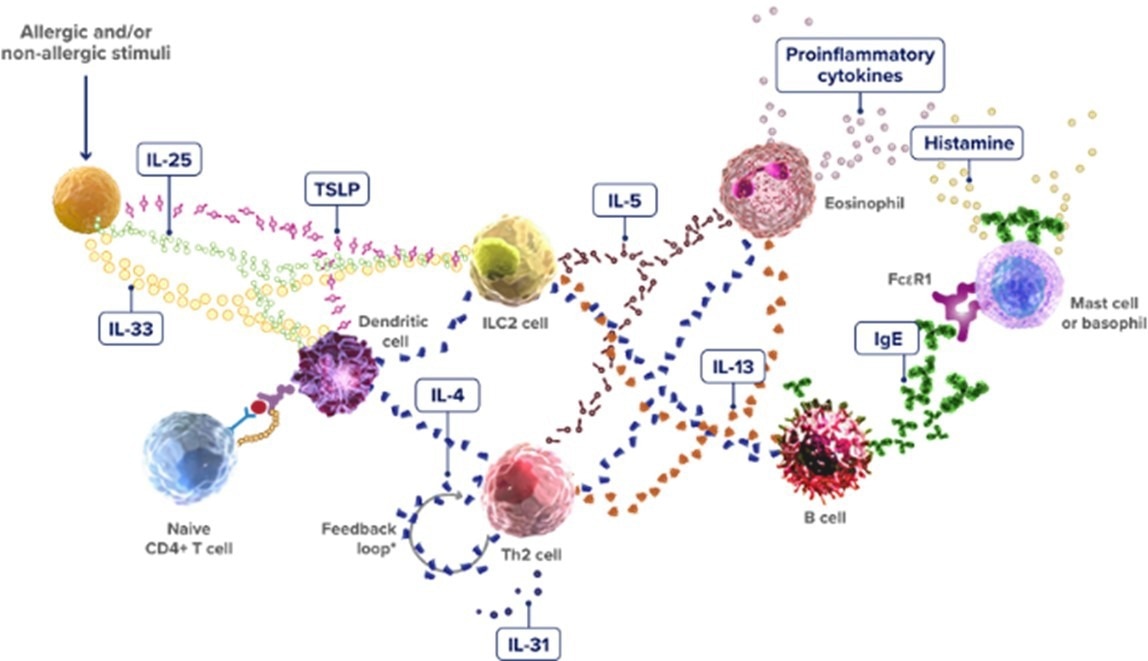
Figure 2. The Type 2 Inflammatory Pathway. Image Credit: https://www.type2inflammation.com/resources/interactive-inflammation-pathway.
Secukinumab targeting IL-17A
Cosentyx® (secukinumab) from Novartis is an innovative biologic. This fully human IgG1/κ monoclonal antibody targets interleukin-17A (IL-17A) and is used in the treatment of diseases such as ankylosing spondylitis, plaque psoriasis, and psoriatic arthritis.
Mechanism of action
The IL-17 family is comprised of pro-inflammatory cytokines able to mediate normal immune and inflammatory responses. IL-17 is primarily produced by T cells, most notably Th17 cells, but IL-17 can also be triggered by neutrophils and mast cells.
IL-17A is often upregulated in autoimmune diseases and has been linked to chronic immune-mediated inflammatory disorders like plaque psoriasis. IL-17A is more potent and boasts a higher affinity for IL-17 receptors than other family members, such as IL-17F.
Secukinumab works by selectively binding to and inhibiting IL-17A, preventing the activation of related pro-inflammatory signaling pathways by blocking its interaction with IL-17 receptors.
Biomarker research in clinical trials
The FDA’s Clinical Pharmacology and Biopharmaceutics Review outlines secukinumab’s pharmacodynamic studies, describing how these studies leveraged biomarkers such as IL-17A, IL-17F, and human beta-defensin-2 (hBD-2) in order to evaluate drug efficacy, measuring their expression at the protein, mRNA, and tissue levels (Figure 3).
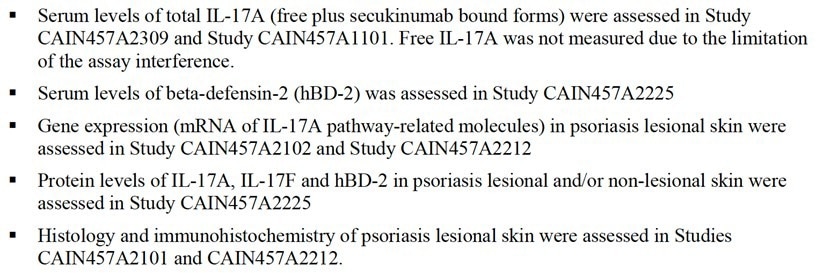
Figure 3. Summary of Clinical Pharmacodynamics. Image Credit: ACROBiosystems
Dupilumab targeting IL-4Rα
Dupixent® (dupilumab) is a fully human IgG4 monoclonal antibody able to bind to the IL-4 receptor, inhibiting its signaling pathway. Dupilumab is an IL-4 receptor α antagonist, blocking the signaling of pro-inflammatory cytokines implicated in atopic and allergic diseases, such as asthma, atopic dermatitis, and sinusitis with nasal polyps.
Mechanism of action
Asthma and other type 2 inflammation in atopic and allergic conditions involve Th2 cell-mediated immune responses. Upregulation of the Th2/type 2 pathway has been noted in a range of inflammatory diseases, with Th2 activation associated with the production of Th2-related cytokines such as IL-4, IL-5, IL-9, and IL-13. IL-4 and IL-13.
These cytokines are key to the regulation of type 2 inflammation, inducing conditions such as asthma, allergic rhinitis, and atopic dermatitis.
IL-4 has two receptor types: Type 1 receptor (IL-4Rα and γc chain) and Type 2 receptor (IL-4Rα and IL-13Rα1).
IL-4Rα is a shared component of both receptor complexes. IL-4Rα is broadly expressed in adaptive and innate immune cells, enabling IL-4 and IL-13 signaling.
Type 1 receptors are principally expressed on lymphocytes, controlling Th2 cell differentiation, while Type 2 receptors are located on resident and myeloid cells.
Dupilumab targets IL-4Rα to inhibit both IL-4 and IL-13 signaling.
Biomarker research in clinical trials
The FDA’s Clinical Pharmacology and Biopharmaceutics Review states that Dupilumab’s pharmacodynamic evaluations involved the use of serum levels of IL-4 and IL-13 as standard PD markers.
Exploratory evidence also showed post-treatment reductions in serum concentrations of thymus and activation-regulated chemokine (TARC/CCL17), total serum IgE, and lactate dehydrogenase (LDH) (Figure 4).
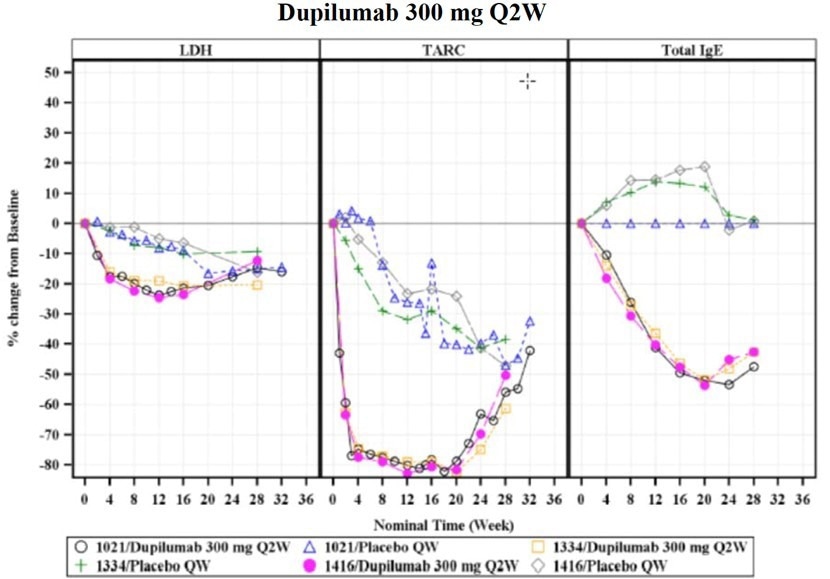
Figure 4. LDH, TARC/CCL17 and IgE changes (in percentage) from baseline under 300 mg Dupilumab treatment once per week, 16 weeks duration. Image Credit: ACROBiosystems
Summary
Biomarkers such as IL-17A and IL-17F are vital across the early research, preclinical, and clinical trial stages for biologics targeting the TNF/IL-23/IL-17 axis. TARC/CCL17 also functions as a key biomarker for biologics aimed at the type 2 inflammatory pathway. Other important biomarkers include cytokines such as IL-12/IL-23p40, IL-23, IL-4, and IL-13.
ACROBiosystems offers a variety of ELISA kits for quantifying biomarkers, cytokines, and other analytes designed to support targeted drug development for autoimmune diseases. These kits have been validated using genuine samples in order to ensure accuracy, sensitivity, specificity, and reproducibility in analytical results.

Product validation data
ClinMax™ Human TARC/CCL17 ELISA Kit (Cat. No. CEA-C029)
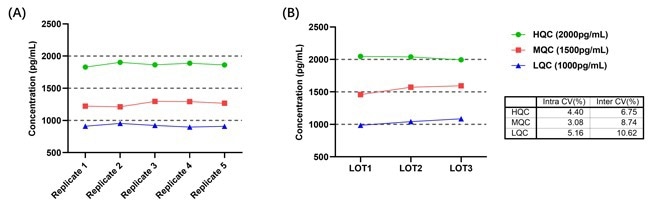
Figure 5. (A) Intra-Assay Precision Data for CEA-C029; (B) Inter-Assay Precision Data for CEA-C029. Image Credit: ACROBiosystems
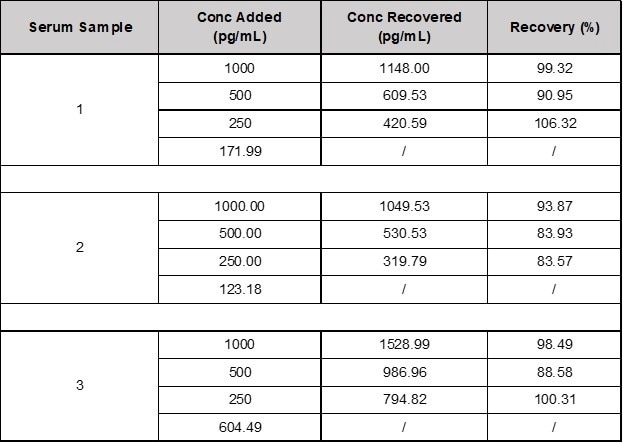
Table 1. Recovery of TARC/CCL17 in Spiked Samples. Source: ACROBiosystems
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.