From the 1980s, therapeutic protein drugs — monoclonal antibodies, proteins, antibody-drug conjugates, bispecific antibodies, etc. — have been widely employed in the treatment of HIV, tumors and other diseases. This contributed to the development of the pharmaceutical industry and human health.
Pharmacokinetic and immunogenicity studies are carried out for the entire life cycle of drug development. Much attention is paid to the study of therapeutic protein drugs, which is now the consensus of the drug administration authorities and medical research.
The establishment of non-clinical analysis and evaluation methods offers early guidance for clinical research. It aids in enhancing the safety and efficiency of drugs.
The NMPA, FDA and other regulatory agencies necessitate that the drug’s efficiency and safety be demonstrated in animals before it enters the clinic. Both preclinical and clinical types of research are needed to analyze the PK of the drug. The FDA also recommends conducting immunogenicity testing in the IND Phase and clinical phase I.
Thus, the establishment of a good PK/ADA assay is vital for the preclinical, clinical analysis and evaluation of drugs.
Pharmacokinetics (PK assay)
Pharmacokinetic (PK) principles incorporate drug absorption, distribution, metabolism and excretion in organisms. Also, differences in blood drug concentration over time can be elaborated with the help of mathematical principles and techniques.
The blood drug concentration-time curve indicates the dynamic mechanism of the blood drug concentration fluctuating with time, reflective of the metabolism of the drug in the body as blood is the medium for the absorption, distribution, metabolism, excretion of drugs and their metabolites in the body.
The concentration of the drugs in different body fluids and tissues retains a specific proportional relationship with the drug concentration in the blood. These indicate that the change of drug concentration in blood is vital as it is the most used sample.
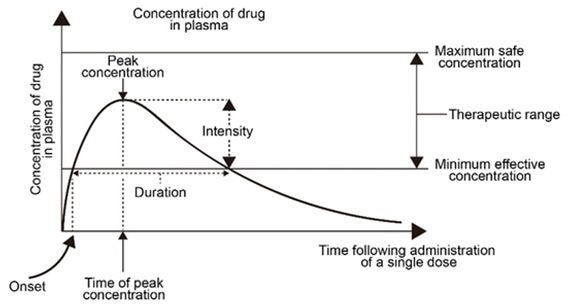
Figure 1. Blood drug concentration-time curve. Image Credit: ACROBiosystems
In pharmacokinetic research, anti-idiotypic antibodies can specifically recognize the unique position of biological drugs and are essential detection reagents for PK studies. They are employed to identify the antibody content of antibody drugs in human or animal serum. PK research performs a vital role in clinical safety, efficiency, analysis of drug interactions, guiding rational drug use and enhancing drug dosage forms.
Anti-drug antibody (ADA assay)
Immunogenicity is defined as the capability of a drug and/or its metabolites to trigger an immune response to itself or related proteins or immune-related events. Immunogenicity has a broad range of effects.
Certain unexpected immune reactions might neutralize the biological activity of drugs or cross immune reactions leading to allergic reactions and cytokine release syndromes. From the clinical manifestation perspective, the immunogenicity of the drug might not have a vital effect on patients, and it may gravely impact the pharmacodynamics, pharmacokinetics, safety and efficacy of the drugs.
Table 1. Immunogenic clinical issues. Source: ACROBiosystems
| Clinical issues |
Clinical results |
| Safety |
Allergic reaction
Autoimmune deficiency |
| Effectiveness |
Increase or decrease drug efficacy
Change the half-life
Change the biological distribution |
| Pharmacokinetics (PK) |
Change PK, PD |
| Other |
Produce antibodies, but no corresponding effect |
Depending on the efficiency and safety hazards of the drug's immunogenicity, the evaluation and monitoring of immunogenicity is an essential research stage in the process of drug development. Immunogenicity research majorly concentrates on the identification and characterization of ADA.
Anti-idiotypic antibody (anti-drug antibody)
Normally, the anti-drug antibody is not a secondary antibody that identifies the constant region of an antibody. An anti-antibody that is hostile to a particular epitope group on the variable region of the antibody molecule is known as an anti-idiotypic antibody.
All biological agents trigger a specific degree of ADA. These antibodies might be harmless and decrease the efficiency and half-life of the drug, and sometimes can be life-threatening.
Anti-idiotypic antibody application
Anti-idiotypic antibodies are broadly employed in drug development and are a vital reference for immunogenicity analysis. It can also help to identify the level of antibody drugs in the body. It is a vital reagent for pharmacokinetics (PK) research.
Table 2. Anti-idiotypic antibody application. Source: ACROBiosystems
| Application |
ADA assay |
PK assay |
| Antibody species |
Rabbit |
Mouse |
| Antibody type |
Polyclonal antibody |
Monoclonal antibody |
| Antibody action |
Positive reference |
Neutralizing antibody: free drug
Non-neutralizing antibody: total drug |
| Sensitivity |
100 ng/mL |
Related to dosage |
Table 3. Selective development of anti-idiotypic antibodies for different drugs. Source: ACROBiosystems
| |
PK assay |
ADA assay |
| Biosimilar drugs |
For the variable region of the drug, development or the original ADA product |
For the variable region of the drug, development or the original ADA product |
| Monoclonal antibody |
Develop single or one pair anti-idiotypic antibody against the variable region of the drug |
Develop a polyclonal antibody against the variable region of the drug |
| Bispecific antibody |
Develop one pair anti-idiotypic antibody against the variable regions of the two parent antibodies |
Develop a polyclonal antibody against the full-length antibody |
| Antibody-Drug Conjugate,ADC |
Develop an anti-idiotypic antibody against the variable region of the drug and the small molecule respectively |
Develop a polyclonal antibody against the entire ADC |
| Nanobody/SCFV |
Develop single or one pair anti-idiotypic antibody against the variable region of the whole antibody |
Develop a polyclonal antibody against the variable region of the whole antibody |
| CAR-T |
For SCFV, Cell-Base Assay |
For SCFV, Cell-Base Assay/ELISA |
ACROBiosystems provides anti-idiotypic antibody development service to help PK/ADA assay of macromolecular drugs
ACROBiosystems concentrates on protein technology, products, and services in the production of biological drugs and is devoted to offering target antigens, other major reagents, and related services necessary for the production of targeted therapeutic drugs.
It offers a series of high affinity, sensitivity, specificity, anti-idiotypic antibodies and PK plasma concentration quantitative detection kits. To satisfy the various needs of the customers, one-stop services from antigen preparation to monoclonal anti-idiotype antibodies, polyclonal anti-idiotype antibodies, pharmacokinetics and immunogenicity testing kit development are provided.
Table 4. Source: ACROBiosystems
| Type |
Anti-ID mouse
monoclonal antibody |
Anti-ID rabbit
polyclonal antibody |
| Scope of application |
PK assay |
ADA assay |
| Preparation cycleM |
4-6 months |
10-14 weeks |
| Advantage |
Single epitope
Good specificity
Good stability between batches |
Can simulate the real situation in blood samples
Relatively short preparation cycle
Low cost |
| General detection method |
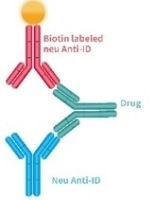 |
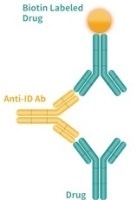 |
Advantages
- Efficient: Rapid response, one-on-one service of the project team, real-time follow-up of the experiment progress
- Professional: Offers major reagents for PK/ADA analysis for numerous drugs (monoclonal antibody, bispecific antibody, ADC, CAR-T)
- Multifunctional: Offers one-stop service from antigen preparation to monoclonal anti-idiotype antibodies, polyclonal anti-idiotype antibodies, PK and immunogenicity test kit developments
- Reliable: Assures delivery of PK/ADA test kits with the sensitivity that meets regulatory requirements
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.