CD19 still remains the strongest contender in CAR-T cell therapy. According to ClinicalTrials.gov, over 600 CAR T cell clinical trials have been carried out to date, a majority of which aiming to treat leukemia or lymphoma patients using CD19‐specific CARs.
CAR-positive T cells are vital to the anti-tumor activity of anti-CD19 CAR T cell products. The percentage of CAR‐positive T cells within a product is often measured using flow cytometry, using anti-Fab antibodies, protein L, or CD19 antigen as detection antibodies. Among these typical choices, the CD19 antigen is widely believed to be the best option, as it offers high specificity and marginal background staining.
ACROBiosystems has produced a wide collection of CD19 antigen products to assist anti-CD19 CAR T investigations, including fluorescent-labeled CD19 and biotinylated CD19 that are uniquely suited for assessing anti-CD19 CAR expression.
Different CAR Detection Strategy and Product Design
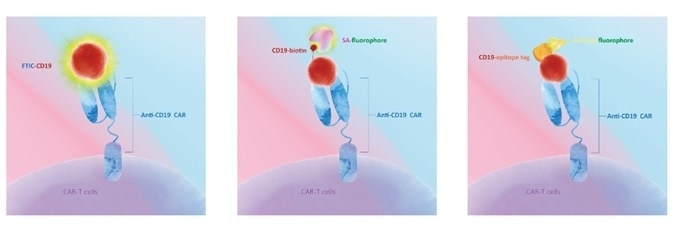
Direct Detection
CD19 comes pre-labeled with FITC. Processing time can be decreased by the use of direct-labeled CD19 protein. A non-specific reaction of a secondary antibody is removed.
| Cat.No. |
Product Description |
Structure |
| CD9-HF2H2 |
FITC-labeled Human CD19 (20-291) Protein |
 |
| CD9-HF251 |
FITC-labeled Human CD19 (20-291) Protein, Fc Tag |
 |
CAR Detection by FITC-Labeled Human CD19, His Tag
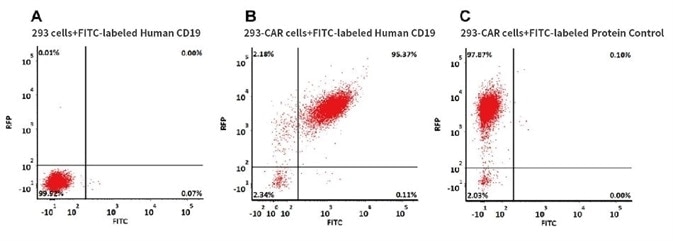
293 cells were transfected with anti-CD19-scFv and RFP tag. 2e5 of the cells were stained with B. FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2, 10 µg/ml) and C. FITC-labeled protein control. A. Non-transfected 293 cells and C. FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2).
CAR Detection by FITC-Labeled Human CD19, Fc Tag
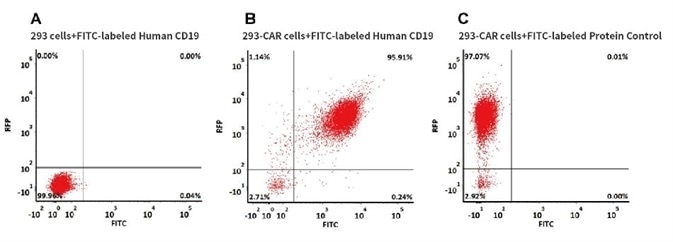
293 cells were transfected with anti-CD19-scFv and RFP tag. 2e5 of the cells were stained with B. FITC-labeled Human CD19 (20-291), Fc Tag (Cat. No. CD9-HF251, 10 µg/ml) and C. FITC-labeled protein control. A. Non-transfected 293 cells and C. FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of FITC-labeled Human CD19 (20-291), Fc Tag (Cat. No. CD9-HF251).
Competitive Advantage over Other Vendors
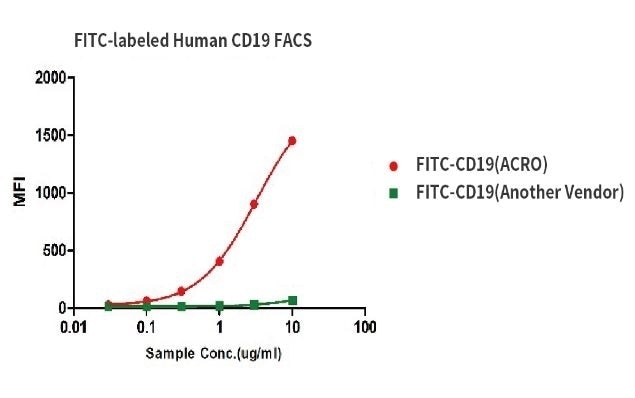
Binding activity of FITC-labeled Human CD19, His Tag from two different vendors were evaluated in the above flow cytometry analysis against anti-CD19-CAR-293 cells. The results show that ACRO’s FITC-labeled Human CD19, His Tag has a much higher binding activity than another vendor’s.
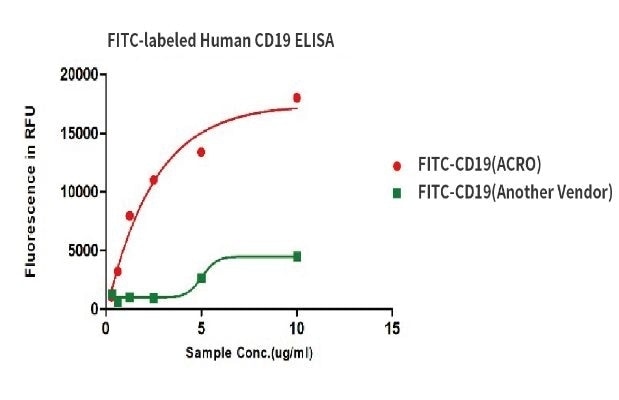
Binding activity of FITC-labeled Human CD19, His Tag from two different vendors were evaluated in the above ELISA analysis against FMC63 MAb. The results show that ACRO’s FITC-labeled Human CD19, His Tag has a much higher binding activity than another Vendor’s.
Well-Controlled Lot Consistency of FITC-Labeled CD19
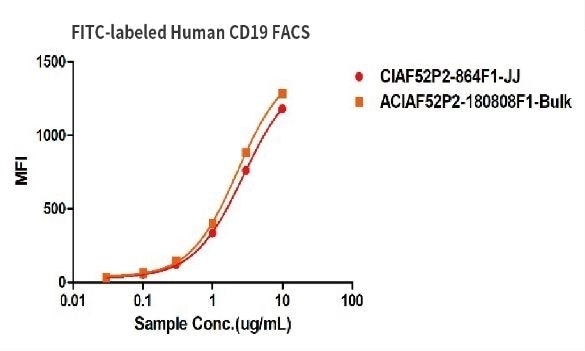
Binding activity of two different lots of FITC-labeled Human CD19, His Tag were evaluated in the above flow cytometry analysis against anti-CD19-CAR-293 cells. The results show that the batch variation among the tested samples is negligible.
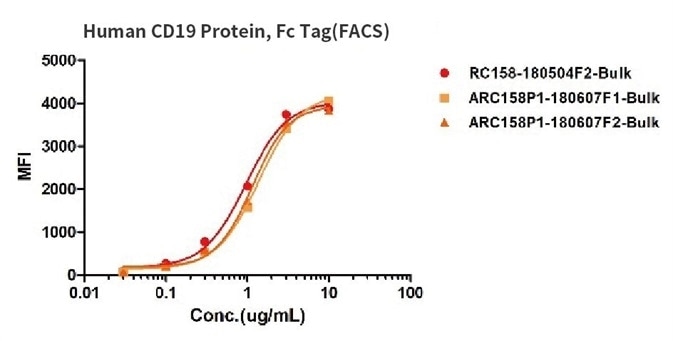
Binding activity of three different lots of FITC-labeled Human CD19, Fc Tag were evaluated in the above flow cytometry analysis against anti-CD19-CAR-293 cells. The results show that the batch variation among the tested samples is negligible.
Consistent Binding Before and After FITC Conjugation
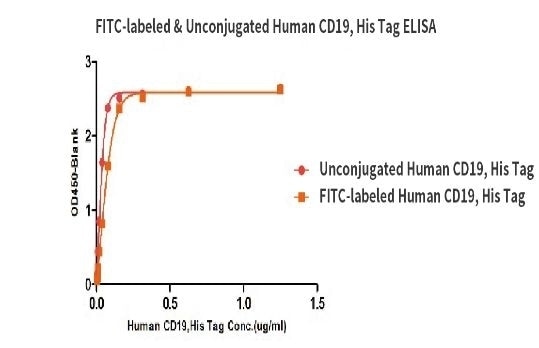
Binding activity of the Human CD19, His Tag before and after FITC labeling were evaluated in the above ELISA analysis. The results show that the FITC-labeled and Unconjugated Human CD19, His Tag have almost the same binding activity.
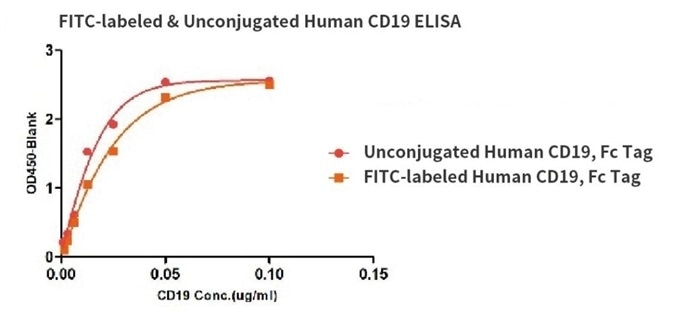
Binding activity of the Human CD19, Fc Tag before and after FITC labeling were evaluated in the above ELISA analysis. The results show that the FITC-labeled and Unconjugated Human CD19, Fc Tag have almost the same binding activity.
Biotin-Streptavidin Based Detection
CD19 comes pre-labeled with biotin and is detected by labeled streptavidin (the biotin-avidin complex). Streptavidin labeled with fluorochromes can bind biotinylated proteins with a high degree of specificity and affinity, intensifying the signal and enhancing the detection sensitivity and specificity.
| Cat.No. |
Product Description |
Structure |
| CD9-H8259 |
Biotinylated Human CD19, Fc Tag, ultra sensitivity (primary amine labeling) |
 |
Indirect Detection by Biotinylated Human CD19, FC Tag
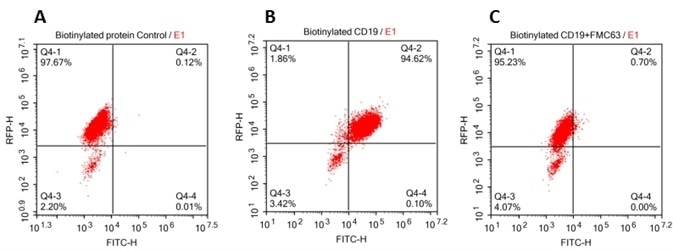
293 cells were transfected with FCM63-scFv and RFP tag. 2x105 of the cells were first incubated with A. Biotinylated protein control. B. Recombinant biotinylated human CD19 (Cat. No. CD9-H8259, 10ug/ml). C. Recombinant biotinylated human CD19 (Cat. No. CD9-H8259, 10 µg/ml) and FMC63(Mouse anti-CD19 antibody). FITC Streptavidin was used to analyze with FACS. RFP was used to evaluate CAR(FMC63-scFv) expression and FITC was used to evaluate the binding activity of recombinant biotinylated human CD19 (Cat. No. CD9-H8259).
Case Study on Real CART Cells
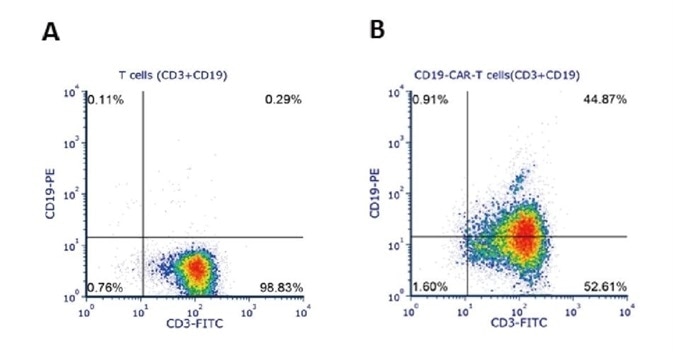
Human T cells were lentivirally transduced with anti-CD19 CAR and cultured for 11 days. Eleven days post-transduction, 1e6 cells were stained for the expression of CD3 and anti-CD19 CAR with FITC anti-human CD3 antibody and biotinylated human CD19 (Cat. No. CD9-H8259) followed by PE-conjugated streptavidin, respectively. A. Non-transduced T cells were used as a control for gating of CAR expression. (Data was kindly provided by Beijing Bowei Huaen Medical Technology Co. Ltd.)
Indirect Detection
CD19 is engineered to carry a specific tag and is detected using a secondary antibody (anti-epitope tag antibody) labeled with a fluorophore. A non-specific reaction of a secondary antibody may occur.
| Cat.No. |
Product Description |
Structure |
| CD9-H52H2 |
Human CD19 (20-291) Protein, His Tag |
 |
| CD9-H5251 |
Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) |
 |
Indirect Detection by Human CD19, His Tag
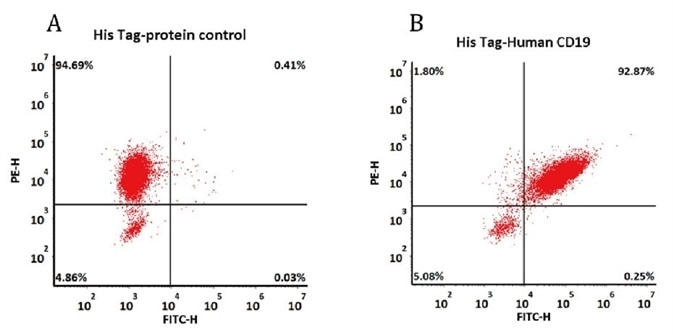
293 cells were transfected with FMC63-scFv and RFP tag. 2e5 of the cells were first incubated with A. His Tag-protein control. B. Recombinant human CD19, His Tag (Cat. No. CD9-H52H2, 10 μg/ml). The FITC Anti-6xHis tag antibody was used to analyze with FACS. RFP was used to evaluate CAR(FMC63-scFv) expression and FITC was used to evaluate the binding activity of recombinant human CD19, His Tag (Cat. No. CD9-H52H2).
Indirect Detection by Human CD19, Fc Tag
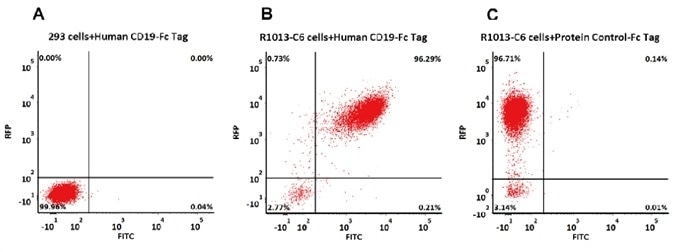
293 cells were transfected with FMC63-scFv and RFP tag. 2e5 of the cells were first stained with B. Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) (Cat. No. CD9-H5251, 3 µg/ml) and C. Human Fc Tag Protein Control, followed by FITC-conjugated Anti-human IgG Fc Antibody. A. Non-transfected 293 cells and C. Human Fc Tag Protein Control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) (Cat. No. CD9-H5251).
Higher Binding Affinity than Competitive Products
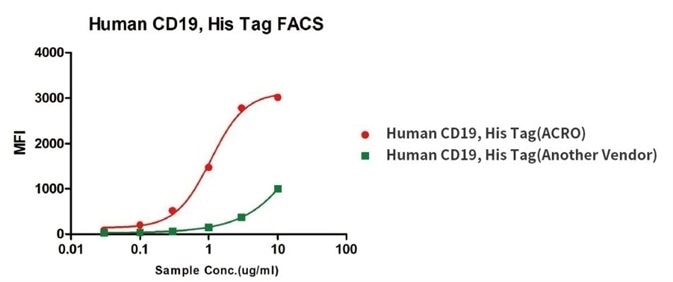
Binding activity of Human CD19, His Tag from two different vendors were evaluated in the above flow cytometry analysis against anti-CD19-CAR-293 cells. The results show that ACRO’s Human CD19, His Tag has a higher binding activity than another Vendor’s.
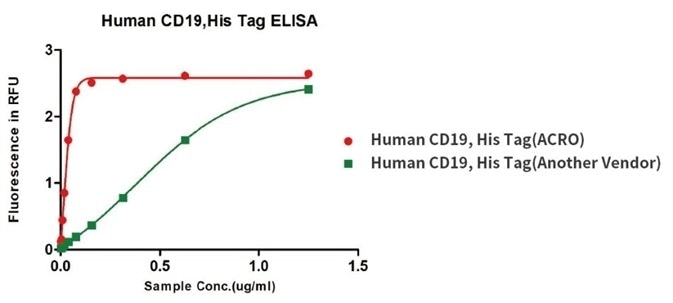
Binding activity of Human CD19, His Tag from two different vendors were evaluated in the above ELISA analysis against FMC63 MAb. The results show that ACRO’s Human CD19, His Tag has a higher binding activity than another Vendor’s.
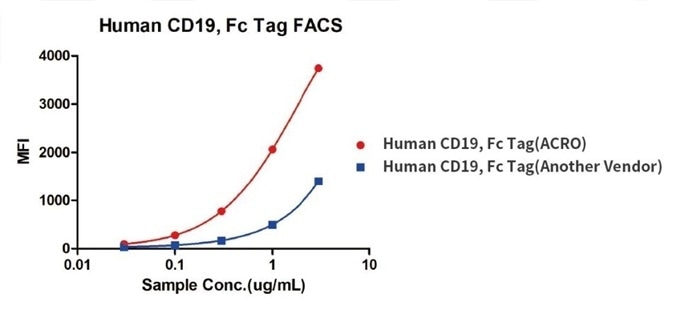
Binding activity of Human CD19, Fc Tag from two different vendors were evaluated in the above flow cytometry analysis against anti-CD19-CAR-293 cells. The results show that ACRO’s Human CD19, Fc Tag has a higher binding activity than another vendor’s.
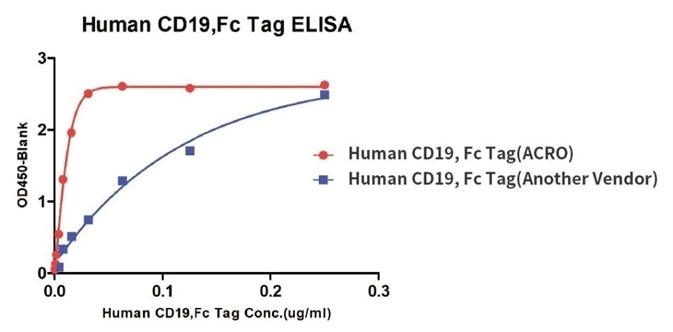
Binding activity of Human CD19, Fc Tag from two different vendors were evaluated in the above ELISA analysis against FMC63 MAb. The results show that ACRO’s Human CD19, Fc Tag has a higher binding activity than another vendor’s.
Higher Purity than Competitive Products
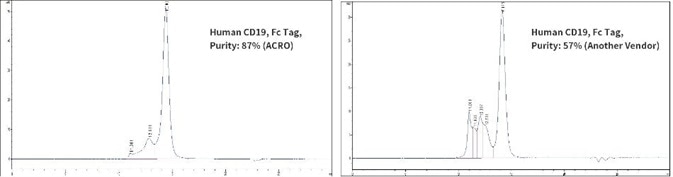
The purity of Human CD19, Fc Tag from two different vendors were determined in HPLC analysis. The results show that the purity of ACRO’s Human CD19, Fc Tag is greater than 87%, while the purity of another vendor’s Human CD19, Fc Tag is greater than 57%.
Better Performance in SPR Studies than Competitive Products
Comparative Analysis of rhCD19-His from ACRO and Another Vendor for Affinity to FMC63 by SPR
Experimental Design
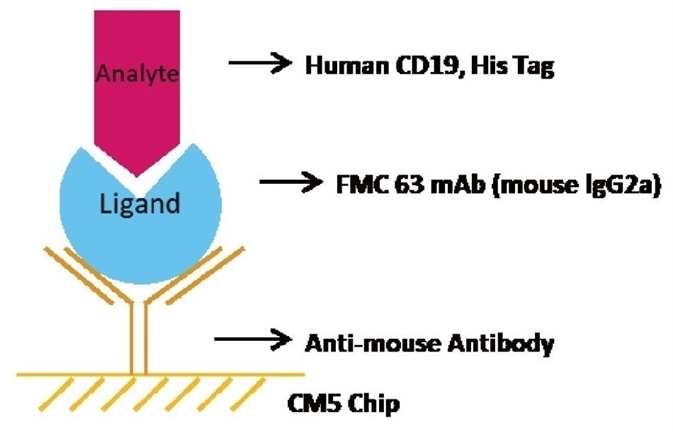
Brief Protocol
- Chip Preparation: The sensor chip CM5 was triggered using EDC/NHS. Immobilization of the anti-mouse antibody took place on the CM5 sensor chip.
- Ligand Capturing: Capture FMC63 mAb to the CM5 chip with immobilized anti-mouse antibody
- Analysis: A series of Human CD19, His Tag concentrations including 0 nM, 5.86 nM, 11.72 nM, 23.44 nM, 46.88 nM, 93.75 nM, 187.5 nM, and 375 nM were injected consecutively, each with a dissociation time of 480 seconds and a contact time of 120 seconds.
- Regeneration: The chip was regenerated with the help of 10 mM Glycine-HCl buffer at PH1.7.
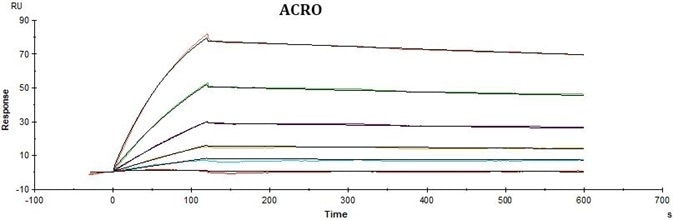
Experimental Results
| Product |
Vendor |
Method |
Ligand |
Capture
Level |
Analyte
Conc. |
Ka
(1/Ms) |
Kd
(1/s) |
KD(M) |
Rmax
(RU) |
% Ligand
Bound |
RU
(375nM) |
Human CD19,
His Tag
(Cat.No. CD9-H52H2) |
ACRO |
Mouse Antibody Capture |
FMC63 |
477 RU |
hCD19 his
12-0.375ug/ml
(240-7.5nM) |
1.02
E + 05 |
3.00
E-04 |
2.95
E-09 |
91.2 |
28.7% |
85 |
Human CD19,
His Tag |
Another Vendor |
N.A. |
N.A. |
N.A. |
29.6 |
9.3% |
6 |
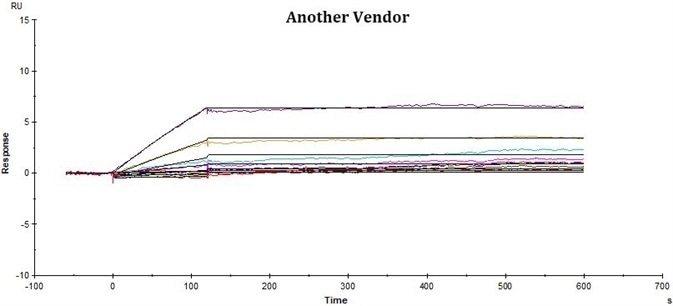
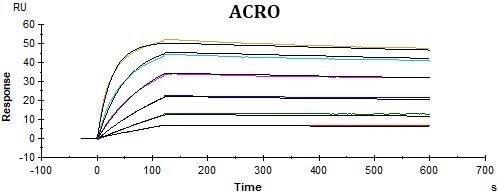
Conclusions
ACRO’s rhCD19-His displayed better performance in SPR assay compared to that of the equivalent from another vendor under the same conditions. The affinity constant (KD value) of ACRO’s rhCD19-His to FMC63 was 2.95 nM, which was consistent with the published data (Nicholson, Ian C. et al.).
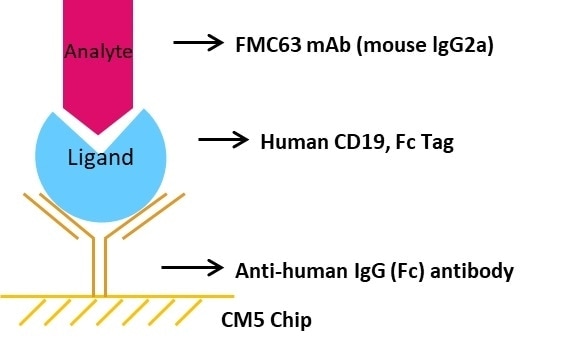
Comparative Analysis of rhCD19-Fc from ACRO and Another Vendor for Affinity to FMC63 by SPR
Experimental Design
Brief Protocol
- Chip Preparation: The sensor chip CM5 was triggered using EDC/NHS. Immobilization of the anti-human IgG (Fc) antibody was done on the CM5 sensor chip.
- Ligand Capturing: Capture Human CD19, Fc Tag to the CM5 chip with immobilized anti-human IgG (Fc) antibody.
- Analysis: A series of FMC63 mAb concentrations including 0 nM, 1.25 nM, 2.5 nM, 5 nM, 10 nM, 20 nM, and 40 nM were injected consecutively, each with a dissociation time of 480 seconds and a contact time of 120 seconds.
- Regeneration: Using 3 M magnesium chloride, the chip was regenerated.
Experimental Results
| Product |
Vendor |
Method |
Ligand |
Capture
Level |
Analyte
Conc. |
Ka
(1/Ms) |
Kd
(1/s) |
KD(M) |
Rmax
(RU) |
% Bound |
Human CD19,
His Tag
(Cat.No. CD9-H5251) |
ACRO |
Anti-human lgG capture |
Fc-CD19 |
220 RU |
FMC63
6-0.19ug/ml
(40-1.25nM) |
950378.1 |
0.00016 |
1.69
E-10 |
50.84 |
17.6% |
Human CD19,
Fc Tag |
Another Vendor |
Fc-CD19 |
2221 RU |
815157.7 |
0.000281 |
3.44
E-10 |
19.20 |
6.6% |
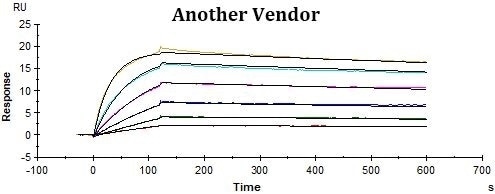
Conclusions
ACRO’s rhCD19-Fc displayed improved performance in SPR assay compared to that of the counterpart from another vendor under the same conditions. The affinity constant (KD value) of ACRO’s rhCD19-Fc to FMC63 was 0.17 nM, which was twice as high as the counterpart from another vendor.
Reference
Nicholson, Ian C. et al. “Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma.” Molecular Immunology, vol. 34, No. 16-17, 1157-1165, 1997.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.