Asthma is a chronic inflammatory condition marked by an imbalance in the immune response within the airways. According to Lancet, asthma affects around 339 million people globally.
While conventional treatments, like inhaled corticosteroids and β-agonists, can ease symptoms, they do not target the underlying pathogenic pathways or change disease progression.
Endotype-based classification (Th2-high vs. Th2-low/non-Th2) has transformed asthma care.
- Th2-type inflammation, caused by eosinophils, TSLP, IgE, IL-4/IL-13, and IL-5, affects 50-70% of patients. Targeted treatments are nearing clinical maturity.
- Non-Th2-type inflammation, which accounts for 30-50% of cases, involves neutrophils, IL-6, and IL-17.
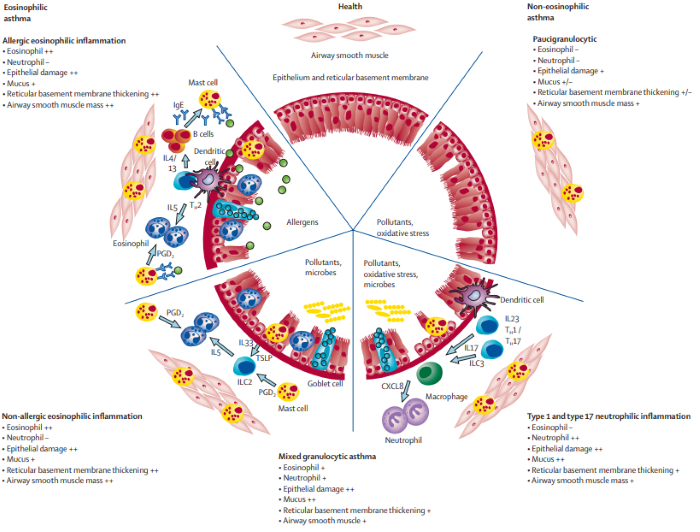
Mechanisms and characteristic pathological features of asthma immunopathology. Image Credit: ACROBiosystems
Th2-associated targets: The central hub of type 2 inflammation
TSLP: The master regulator of the inflammatory cascade
Airway epithelial cells release TSLP in response to inflammatory stimuli, which activates dendritic cells, induces Th2 immunological polarization, and initiates the IL-4/IL-5/IL-13 cytokine cascade.
The responses combine to form a self-amplifying "inflammatory storm".
Tezepelumab (Amgen/AstraZeneca)
This monoclonal antibody blocks TSLP signaling and has been shown to reduce annual asthma exacerbations by 72% in patients with severe asthma.
SHR-1905 (Hengrui)
Currently in Phase II clinical trials, this candidate targets both asthma and chronic rhinosinusitis with nasal polyps (CRSwNP).
CM326 (Keymed biosciences)
With an epitope-optimized design and threefold affinity increase, CM326 is being used in combination therapy trials with omalizumab. Regulatory submission is expected by 2025.
With new indications for atopic dermatitis and eosinophilic esophagitis, the global market for TSLP inhibitors is projected to reach nearly $8 billion by 2030, positioning these therapies as a leading approach in type 2 inflammation management.
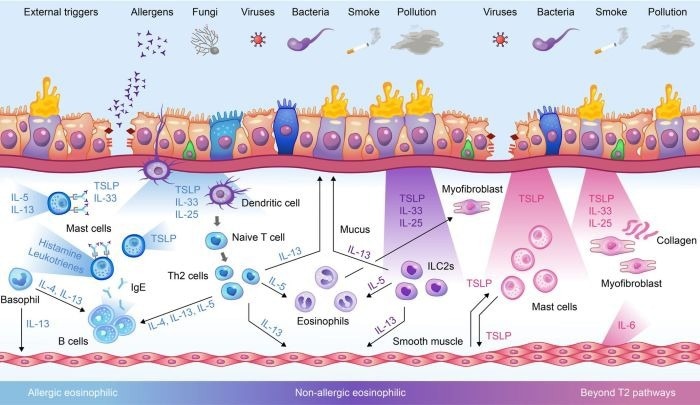
The role of TSLP in driving asthma disease mechanisms via different inflammatory pathways. Image Credit: ACROBiosystems
IL-4/IL-13: The synergistic duo in dual-target therapeutics
IL-4 stimulates IgE synthesis and Th2 polarization, whereas IL-13 promotes mucus hypersecretion and fibrotic remodeling. Targeting both cytokines simultaneously can dampen type 2 inflammation more effectively by disrupting complementary disease mechanisms.
Dupilumab (Sanofi) cemented its position as the top-selling inflammatory illness medication with its first-in-class IL-4Rα antagonist. In 2024, it generated $14.179 billion in sales (23% YoY growth).
AK139 (Consonance Biologics) is a bispecific antibody that targets IL-4/IL-13 (via IL-4Rα blockage) and ST2 pathway activation, achieving triple-action (IL-4/IL-13/ST2 inhibition) to address both dupilumab-responsive and refractory disorders, such as hypereosinophilic syndrome (HES).
This mechanism establishes AK139 as a possible paradigm-shifting therapy for complicated type 2 inflammatory disorders, surpassing the limitations of single-pathway inhibitors.
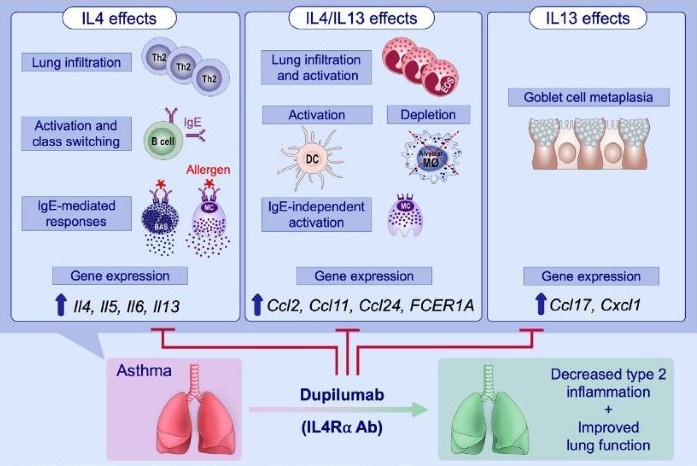
Dual blockade of IL-4 and IL-13 with dupilumab. Image Credit: ACROBiosystems
IL-5/IL-5RA: A triple-targeted strike against eosinophilic pathogenesis
IL-5 and its receptor IL-5RA are part of the central signaling axis in eosinophil survival, differentiation, and activation. They drive inflammation and tissue damage in conditions like severe asthma, EGPA, and HES.
As the first anti-IL-5 mAb, Mepolizumab (GSK) inhibits IL-5/IL-5RA interaction and lowers eosinophil counts in severe asthma/EGPA.
Benralizumab (AstraZeneca) is an anti-IL-5RA mAb. It induces antibody-dependent cellular cytotoxicity (ADCC), rapidly depleting eosinophils in asthma/CRSwNP.
Reslizumab (Teva) neutralizes circulating IL-5 and is licensed for refractory eosinophilic asthma with increased eosinophils.
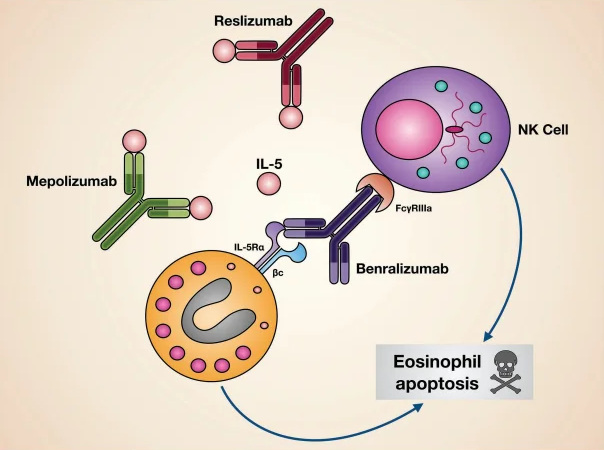
Mechanisms of action of biological drugs targeting IL-5 or its receptor. Image Credit: ACROBiosystems
IgE: The upstream orchestrator of allergen-driven hypersensitivity
IgE, the least abundant antibody isotype in human serum, exerts its effects through two receptors: FcεRI, found on mast cells and basophils, and FcεRII (CD23), primarily expressed on B cells and eosinophils.
When free IgE binds to FcεRI and is crosslinked by allergens, it triggers mast cell and basophil degranulation, releasing histamine, leukotrienes, and other inflammatory mediators that drive acute allergic reactions.
First-in-class monoclonal antibody Omalizumab (Genentech/Novartis) neutralizes free IgE and prevents FcεRI/FcεRII binding. It remains a standard treatment for moderate-to-severe allergic asthma and chronic spontaneous urticaria (CSU), achieving long-term suppression of IgE-mediated airway inflammation.
Ligelizumab (Novartis) and LP-003 (Tiancheng Biologics) are high-affinity anti-IgE monoclonal antibodies in Phase III trials for IgE-dependent diseases. Preclinical studies suggest these candidates may outperform Omalizumab in suppressing IgE activity.
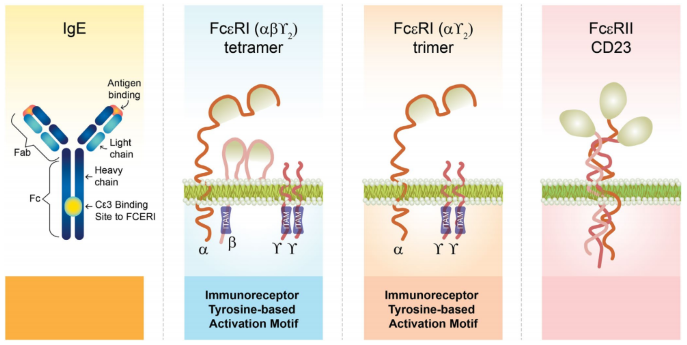
The structure of IgE and IgE receptors. Image Credit: ACROBiosystems
IL-33/ST2 axis: An alarm amplifier for tissue injury
The IL-33/ST2 axis is a key driver of asthmatic airway inflammation. Airway epithelial cells release IL-33 in response to allergens or tissue injury, activating innate lymphoid cells (ILC2s) and Th2 cells.
These effector cells produce mediators, including IL-5 and IL-13, causing eosinophilic infiltration, mucus hypersecretion, and airway hyperresponsiveness. This results in a pro-inflammatory positive feedback loop.
Clinical data suggest that sputum or serum IL-33 levels correspond with illness severity in asthma patients. Soluble ST2 (sST2) may operate as an indirect biomarker of pathway activation.
Etokimab (I-Mab Biopharma), which is optimized for common IL-33 gene polymorphisms, has completed patient recruitment in its Phase II trial. The strategic combination of IL-33 inhibitors with TSLP-targeted treatments via "dual upstream blockade" shows promise in expanding therapeutic coverage to broader patient populations.
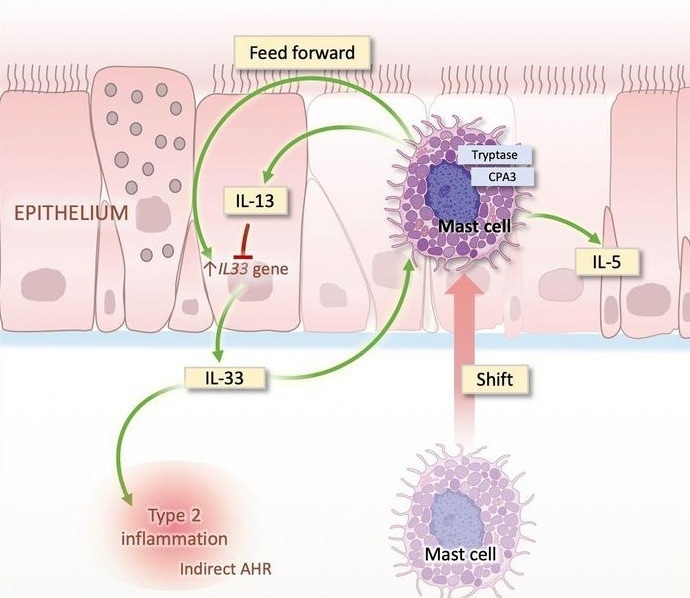
Airway epithelium–shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. Image Credit: ACROBiosystems
Non-Th2 targets: Illuminating the hidden frontier in tackling refractory asthma
Traditional asthma therapies are largely aimed at Th2-driven inflammation. However, nearly half of asthma patients don't respond adequately to these treatments and are categorized as having “refractory asthma”.
In recent years, research has discovered that non-Th2 inflammatory processes, including Th1, Th17, and neutrophilic inflammation, play important roles in refractory asthma.
As new studies continue to advance the understanding of asthma, new avenues are emerging to help overcome existing treatment barriers.
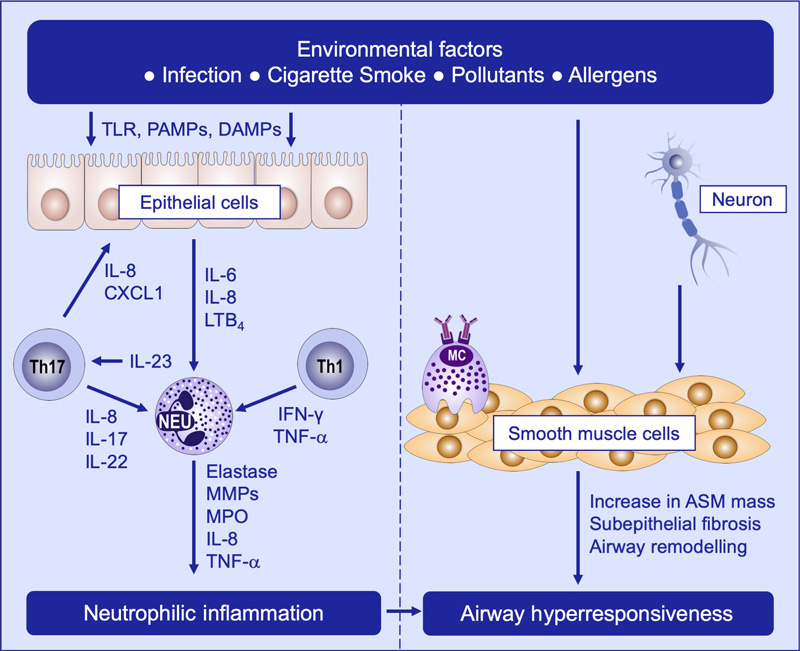
Mechanisms of non-T2 asthma, including neutrophilic inflammation and airway hyperresponsiveness. Image Credit: ACROBiosystems
Key cytokines associated with non-Th2 asthma: IL-17, IL-8, and IL-6
IL-17 activates bronchial epithelial cells to release IL-6 and IL-8 through p38 MAP kinase and ERK phosphorylation pathways, leading to neutrophil recruitment in the airways.
Elevated IL-17 levels in severe asthma patients' sputum and serum have been found to act as an independent illness risk factor.
Airway epithelial cells, T cells, macrophages, and activated neutrophils release the most potent pulmonary neutrophil chemokine, IL-8.
Sputum IL-8 levels are significantly higher in non-eosinophilic asthma patients than in eosinophilic phenotypes and healthy controls. These levels correlate positively with sputum neutrophil proportion and count, indicating a unique role in non-eosinophilic asthma pathology via a positive feedback loop.
Asthma patients also have elevated levels of IL-6 in their serum, BAL fluid, and sputum, which correlates adversely with lung function.
A high-IL-6 phenotype, associated with obesity, metabolic problems, mixed granulocytic inflammation, and a poorer prognosis, may also be applicable for use as a biomarker for asthma endotyping.
Solutions of ACROBiosystems: Empowering asthma drug development
To support the development of targeted asthma drugs and therapies, ACROBiosystems have created a variety of products targeting core mechanisms in asthma pathology, such as recombinant proteins, cell lines, and inhibitor screening kits for TSLP/TSLPR, IL-4/IL-4R, IL-5/IL-5R, IL-13/IL-13R, IL-6/IL-6R, IL-8, IL-17/IL-17R, IL-33, and IgE Fc.
Image Credit: ACROBiosystems
Combox, universal products recommendation

Streptavidin (SA) Series Products. Image Credit: ACROBiosystems

Isotype Controls. Image Credit: ACROBiosystems
Universal Antibodies. Image Credit: ACROBiosystems
References
- Busse, W.W. (2019). Biological treatments for severe asthma: A major advance in asthma care. Allergology International, 68(2). https://doi.org/10.1016/j.alit.2019.01.004.
- Caminati, M., et al. (2023). Tezepelumab in patients with allergic and eosinophilic asthma. Allergy, 79(5), pp.1134–1145. https://onlinelibrary.wiley.com/doi/10.1111/all.15986.
- Le Floc’h, A., et al. (2020). Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy, (online) 75(5), pp.1188–1204. https://doi.org/10.1111/all.14151.
- Pelaia, C., et al. (2019). Interleukin-5 in the Pathophysiology of Severe Asthma. Frontiers in Physiology, 10(1514). https://doi.org/10.3389/fphys.2019.01514.
- Domingo, C., et al. (2025). The Direct and Indirect Role of IgE on Airway Epithelium in Asthma. Allergy. https://doi.org/10.1111/all.16459.
- Altman, M.C., et al. (2020). Airway epithelium–shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. The Journal of Clinical Investigation, (online) 129(11), pp.4979–4991. https://doi.org/10.1172/JCI126402.
- Sze, E., Bhalla, A. and Nair, P. (2019). Mechanisms and therapeutic strategies for non‐T2 asthma. Allergy, 75(2), pp.311–325. https://doi.org/10.1111/all.13985.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.