Tau protein is a microtubule-associated protein, widely present in the central nervous system. It plays a key role in maintaining and regulating the structure and function of neurons.
In neurodegenerative diseases, the abnormal aggregation of Tau proteins leads to the formation of neurofibrillary tangles. These tangles disrupt neuronal integrity and contribute to cell death and disease progression.
To develop new diagnostic methods and therapeutic approaches to treat these neurodegenerative diseases, investigation into the role of Tau protein and its subsequent aggregation and formation of neurofibrillary tangles is essential.
The critical role of Tau protein in the neuronal cytoskeleton
Neurons are complex cells whose functions and structures depend on the dynamic reorganization of the cytoskeleton.
The cytoskeleton helps cells maintain their shape and internal organization, individually carried out by individual elements within it. Microfilaments resist tension, microtubules maintain cell shape and facilitate cargo transport, while intermediate filaments anchor organelles.
Tau protein works by binding to microtubules in axons, promoting their assembly and preventing depolymerization, and enhancing stability.
Tau's function is finely regulated by post-translational modifications, especially phosphorylation, which decreases the proteins affinity for microtubules and affects their dynamics. This process is essential in healthy neuronal function.
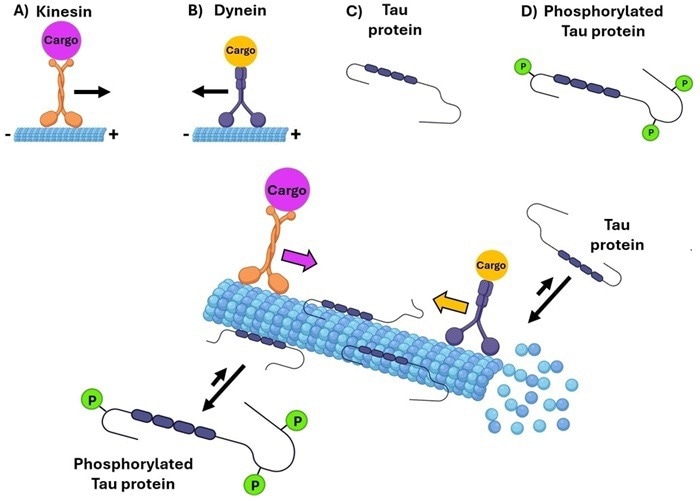
Figure 1. Tau protein's role in microtubule stability and transport. Image Credit: https://doi.org/10.1002/cmdc.202400180
Structural features of Tau protein
Tau proteins consist of four primary domains: the N-terminal projection domain, proline-rich region, microtubule-binding domain (MBD), and C-terminal region.
The N-terminal domain contributes to axonal stability by interacting with other cytoskeletal components and cell membranes.
The proline-rich region contains phosphorylation sites that regulate neural signaling and intracellular communication.
The MBD determines Tau's binding affinity to microtubules, while the C-terminal region supports the MBD in maintaining microtubule stability and function.
Alternative splicing produces six Tau isoforms with different N-terminal inserts (0N, 1N, 2N) and MBD repeats (3R or 4R). Each isoform has distinct functionalities, particularly in their ability to bind to microtubules.
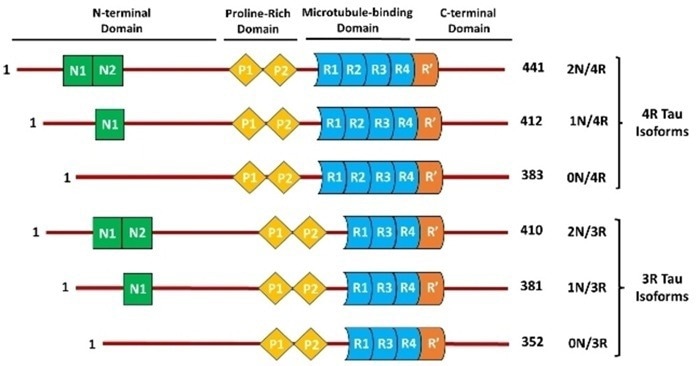
Figure 2. Six Tau isoforms are generated by alternative splicing. Image Credit: https://doi.org/10.1002/cmdc.202400180
Tau protein and neurodegenerative diseases
Tau protein in Alzheimer's disease
Alzheimer’s Disease (AD) is the most prevalent neurodegenerative disorder among the elderly, marked by abnormal Tau phosphorylation. Phosphorylated Tau proteins detach from microtubules, aggregating into neurofibrillary tangles and disrupting neuronal structures. This disruption leads to AD’s distinctive symptoms of memory loss and cognitive decline.
Amyloid-beta (Aβ) accumulation is also strongly characteristic of AD. Research indicates a strong correlation between Tau pathology and Aβ accumulation.
In the early stages of AD, Aβ plaques form between neurons, triggering inflammation and oxidative stress. This promotes Tau phosphorylation and aggregation, which can lead to exacerbated Aβ toxicity, causing a detrimental cycle that accelerates AD progression.
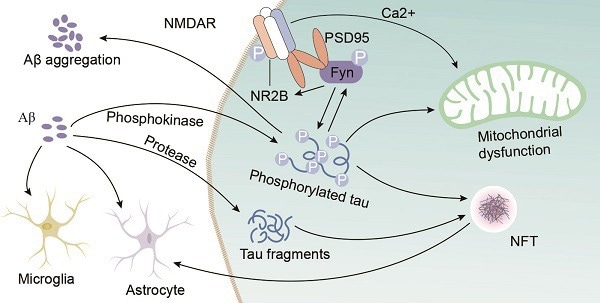
Figure 3. Interaction of Aβ and Tau in AD. Image Credit: https://doi.org/10.7150/ijbs.57078
Tau protein in Parkinson's disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease, primarily characterized by the abnormal aggregation of α-synuclein (α-syn) into Lewy bodies. This aggregation results in the progressive loss of dopaminergic neurons in the substantia nigra.
Emerging evidence suggests that Tau protein also contributes to PD pathology.
Research has shown that Tau interacts with α-syn, accelerating its aggregation. Pre-formed fibrils (PFFs) of mixed Tau and α-syn demonstrate stronger propagation activity than α-syn PFFs alone, inducing mitochondrial dysfunction, synaptic impairment, and neurotoxicity. Autopsy studies in PD patients add evidence to this idea of Tau and α-syn co-localization in Lewy bodies.
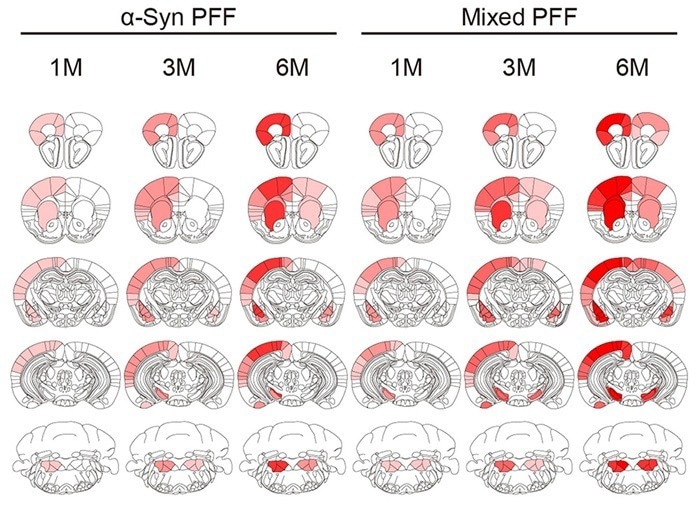
Figure 4. Heatmap of pS129 pathology scores in mouse brains injected with different PFFs. Image Credit: https://doi.org/10.1093/brain/awac171
pS129 is a phosphorylation site on α-syn and a marker of α-syn aggregation. The heatmap shows the distribution and severity of α-syn pathology across brain regions in mice injected with α-syn PFFs or mixed Tau/α-syn PFFs. Redder areas indicate more severe pathology, with mixed Tau/α-syn PFFs causing broader and more severe α-syn pathology.
Tau protein in Huntington’s Disease (HD)
Huntington’s Disease (HD) is another common neurodegenerative disorder. Unlike AD and PD, HD is caused by mutations in the huntingtin (HTT) gene. It is characterized by motor dysfunction, cognitive decline, and psychiatric symptoms.
Although Tau phosphorylation is not an indicator of HD, abnormal Tau phosphorylation and aggregation have been observed in the disease's later stages. Mutant huntingtin protein (mHtt) may disrupt Tau regulation, leading to this observed phosphorylation and aggregation.
Altered 4R/3R Tau isoform ratios in the cortex and striatum of HD patients also suggest that the mis-splicing of Tau is associated with HD pathology.
Summary
Tau protein is essential for microtubule stability and neuronal function. The protein’s disfunction is linked to several neurodegenerative diseases, such as AD, PD, and HD.
To support research into Tau mechanisms and related diseases, AcroBiosystems has a diverse range of high-quality Tau products available, including wild-type, mutant, and phosphorylated Tau proteins, Tau PFFs, Tau stable cell lines, and p-tau antibodies. These AcroBiosystems products cater to the varied needs of basic research and drug development.
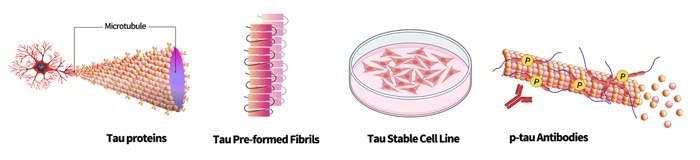
Image Credit: ACROBiosystems

Image Credit: ACROBiosystems

Image Credit: ACROBiosystems
References
- Di Lorenzo, D. (2024). Tau Protein and Tauopathies: Exploring Tau Protein–Protein and Microtubule Interactions, Cross‐Interactions and Therapeutic Strategies. ChemMedChem, 19(21). https://doi.org/10.1002/cmdc.202400180.
- Yang, J., Zhi, W. and Wang, L. (2024). Role of Tau Protein in Neurodegenerative Diseases and Development of Its Targeted Drugs: A Literature Review. Molecules, (online) 29(12), pp.2812–2812. https://doi.org/10.3390/molecules29122812.
- Zhang, H., et al. (2021). Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. International Journal of Biological Sciences, (online) 17(9), pp.2181–2192. https://doi.org/10.7150/ijbs.57078.
- Pan, L., et al. (2022). Tau accelerates α-synuclein aggregation and spreading in Parkinson’s disease. Brain: A Journal of Neurology, (online) 145(10), pp.3454–3471. https://doi.org/10.1093/brain/awac171.
- Singh, S., et al. (2025). Targeting tau in Alzheimer’s and beyond: Insights into pathology and therapeutic strategies. Ageing Research Reviews, 104, p.102639. https://doi.org/10.1016/j.arr.2024.102639.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.