The coronavirus pandemic has significantly impacted our daily lives. As of September 20th, over 30 million people have caught the disease, and approximately 1000,000 people have died worldwide.1
The virus has a number of key characteristics. It has an incubation period of up to 14 days, and it can be transmitted during the pre-symptomatic phase. A relatively small asymptomatic population coupled with slow mobilization of diagnostic testing has led to several countries imposing national lockdowns in order to limit the spread of the virus.1,2
These measures have helped lower the number of cases and reduce the reproduction number, leading to many of these countries later lifting their lockdown restrictions.
Unfortunately, because there is currently no available vaccine for SARS-CoV-2, lifting these restrictions may in fact lead to new outbreaks – this scenario has already been seen in Germany.3
Accurate diagnosis of SARS-CoV-2 is therefore vital if new outbreaks are to be identified and timely quarantine measures are to be implemented to limit the spread of infection, ultimately eliminating the virus.4,5
Diagnostic tests for COVID-19
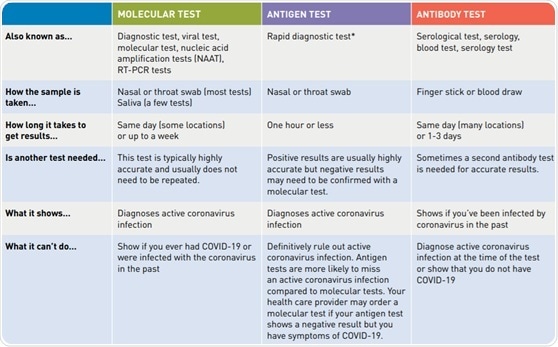
Three main types of diagnostic tests are available for the identification of SARS-CoV-2: the molecular test, the antibody test, and the antigen test. The molecular and antigen test are both capable of identifying the presence of an active coronavirus infection, and the antibody test is able to confirm whether or not a patient has previously been infected with SARS-CoV-2.11
Due to the body’s immunological response, antibody tests have been able to diagnose patients up to 35 days after COVID-19 symptoms have developed.7 The table below displays a comparison of these three different tests.
Numerous existing testing methodologies including reverse transcription-polymerase chain reactions (RT-PCR) employ an RNA template for the detection of SARS-CoV-2 genes, however, many of these approaches are inconvenient and time-consuming.
Antibody and antigen detection of COVID-19 infection is more efficient, allowing for faster diagnostics with some kits even approaching instantaneous detection.11
The critical role of antigens and antibodies in SARS-CoV-2 rapid diagnosis
By August 28th, the FDA had authorized 194 diagnostic tests for emergency use. These tests employ three different detection methods, including 4 antigen tests and 40 antibody tests.4,6,8 Unfortunately, a combination of urgent demand and the relaxation of FDA guidelines has resulted in some of these tests demonstrating poor performance.7,8
One of the primary interferences of immunoassay performance is cross-reactivity. The two key proteins used in SARS-CoV-2 serological tests – the spike (S) glycoprotein and the nucleocapsid (N) protein – include immunogenic domains conserved in strains of other β-coronaviruses. Some of which are known to cause the common cold.2
Highly specific antibody and antigen reagents are therefore essential, and kit testing should be performed with high-purity control antibodies to guarantee accuracy while avoiding erroneous results.9,15
Zhao et al. utilized ACROBiosystems’ biotin-labeled S1 protein in the development of a COVID-19 chemiluminescent immunoassay. The test was able to achieve a sensitivity of 93.2% and a specificity of 100% by successfully assessing the total antibody, rather than investigating immunoglobulin (Ig)G or IgM concentration.
This kit was also able to detect SARS-CoV-2 in some patients with undetectable v levels of viral RNA, during the early stages of the illness (within 1 week). In combination with the molecular test, the kit was able to increase the sensitivity for detecting COVID-19 (p<0.001).5
As well as protein reagents, the global impact of COVID-19 requires these tests to be rapidly scalable while delivering high-throughput analysis. They must also be effectively distributed worldwide.
In April, scientists based at the Policlinico San Matteo Hospital in Italy were able to develop a fully automated test, able to analyze up to 170 samples an hour. This chemiluminescent assay detected SARS-CoV-2 IgG antibodies against the S1 and S2 domain of the S protein and was very specific.10
ACROBiosystems offers high-quality antigens and antibodies
ACROBiosystems is at the forefront of recombinant protein manufacture, having already developed over a hundred products to support research aiming to combat SARS-CoV-2.
These products including a series of high-quality antibodies and antigens, designed to assist in the development of SARS-CoV-2 serological diagnostic tests. Table 1 provides a summary of these products.9
Table 1. SARS-CoV-2 products from ACROBiosystems.9
| Product type |
Description |
Options |
| Recombinant proteins |
SARS-CoV-2 related proteins including angiotensin-converting enzyme 2 (ACE2), S, S1, S2, and N proteins harvested from human embryonic kidney 293 cells. |
|
| Antibodies |
Matched antibody pairs for antigen detection and antibodies derived from the serum of convalescent patients for use as a control in assay development. |
|
| Kits |
Kits using antigen or antibody products to screen anti-SARS-CoV-2 drugs and measure antibody or antigen titer. |
|
| Beads |
Protein pre-coupled magnetic beads for increasing the screening efficiency of COVID-19 neutralizing antibodies. |
|
Hot products
Matched antibody pairs for antigen detection:
- Super affinity of 10 pM level (BLI)
- High sensitivity of 12 pg/mL (ELISA)
- High specificity: no cross-reactivity with other coronaviruses
- Suitable for different antigen detection methods, such as CLIA, ELISA, GICA, etc.
- High batch-to-batch consistency and bulk order available
Antigens for antibody detection:
- Customized formulation and bulk order available
- Validated functionality
- High purity and batch-to-batch consistency
These products are scalable and can be distributed globally.9
References and Further Reading
- ECDC.eu (2020). COVID-19 Situation Update Worldwide, as of 28 June 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases.
- Welssleder R., et al. (2020). COVID-19 Diagnostics in Context. Science Translational Medicine.
- BBC.co.uk. (2020). Coronavirus: German Outbreak Sparks Fresh Local Lockdowns. https://www.bbc.co.uk/news/world-europe-53149762.
- ACROBiosystems.com (2020). Specially Designed Proteins for COVID-19 Serological Test Development.
- Zhao J., et al. (2020). Antibody Responses to SARS-CoV-2 in Patients of Novel Coronavirus Disease 2019. Clinical Infectious Diseases.
- ACROBiosystems.com (2020). Serological Tests are Unreliable? The Protein Reagents are the Key. https://www.acrobiosystems.com/A1127-Serological-tests-are-unreliable-The-protein-reagents-are-the-key.html.
- Deeks J.J., et al. (2020). Antibody Tests for Identification of Current and Past Infection with SARS-CoV-2. Cochrane Database of Systematic Reviews.
- ACROBiosystems.com (2020). How Does the COVID-19 Antibody ELISA Test Function? https://www.acrobiosystems.com/A1125-How-does-the-COVID-19-antibody-ELISA-test-function.html.
- ACROBiosystems.com (2020). SARS-CoV-2 Related Products. https://www.acrobiosystems.com/A1111-2019-nCoV-Proteins.html
- Younes N., et al. (2020). Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses.
- FDA.gov (2020). Coronavirus Testing Basics. https://www.fda.gov/
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.