Claudin 6 (CLDN6) is an intercellular adhesion molecule that develops tight junctions all around cells and controls epidermal permeability, restricting the free passage of solutes and water via cells.
Claudin 6 overexpression in the epidermis causes defective permeability barrier function in homozygous mice, resulting in dehydration and death within 48 hours of birth. Claudin 6 is a cofactor for hepatitis C virus (HCV) entry, and overexpression of the protein in 293 T cells conferred susceptibility to HCV entry.
Earlier studies discovered that Claudin 6 was not detected in normal adult tissues. However, it was highly expressed in a range of solid tumor tissues like testicular cancer, ovarian cancer, and endometrial cancer. As a result, Claudin 6 is likely to follow Claudin 18.2, another Claudin family member that could be used to treat cancer.
.
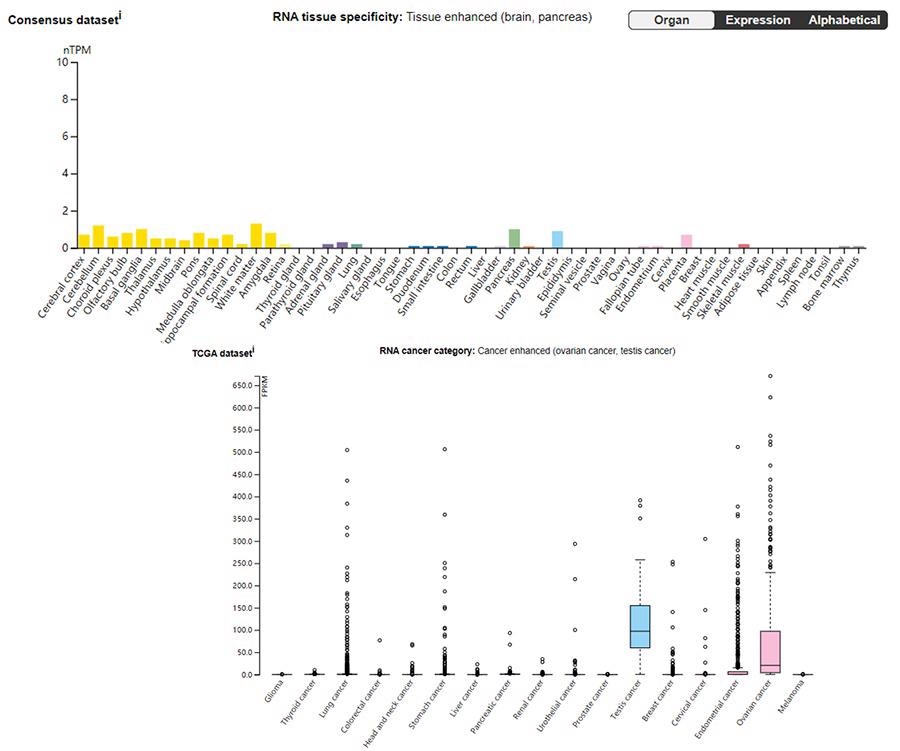
mRNA expression of Claudin 6 in human normal tissue (top) and cancer (bottom). Image from reference
Currently, drug research targeting Claudin 6 is displaying a scene of competition for discoveries.
Monoclonal antibody (mAb)
Ganymed was purchased by Astellas for $1.4 billion in 2016. Apart from being optimistic about IMAB362, which targets Claudin 18.2, and broadening its immuno-oncology pipeline, IMAB027 (ASP1650), which targets Claudin 6, is also significant.
IMAB027 binds to Claudin 6 specifically in preclinical studies, and as a single agent induces Claudin 6+ tumor cell death via ADCC and CDC, resulting in antitumor activity in vitro and in vivo.
Moreover, heterogeneous expression of Claudin 6 in tumors, chemotherapy sensitized cells to ADCC inducing by IMAB027, and IMAB027 in combination with chemotherapeutic agents could optimize the antitumor effect
A Phase I clinical trial in advanced ovarian cancer patients was completed (NCT02054351). In October 2020, a Phase II clinical trial was completed, but it was halted because the primary endpoint was not met.
Anti-Claudin 6 mAb GB-7008, developed by GENCHEM (Shanghai) and I-MAB Biopharma, is in preclinical development for testicular tumors and ovarian cancer.
Antibody-drug conjugate (ADC)
Antibody–drug conjugates (ADCs) are immunoconjugates. They include a monoclonal antibody bound to a cytotoxic drug (called the payload) through a chemical linker. Tumor cell line plasticity is the main mechanism of tumor recurrence or therapeutic resistance.
To avoid drug toxicity, highly plastic tumor cells can be phenotypically transformed to a drug-resistant state. Guangzhou Medical University’s Affiliated Cancer Hospital and Research Institute compared the plasticity of a hepatocellular carcinoma (HCC) cell line using Claudin 6 as a therapeutic target. As a result, a novel anti-Claudin 6 ADC with DM1 as a payload was created.
Preclinical data in HCC cell lines and primary tumors display that anti-Claudin 6 ADC includes potent antitumor efficacy in HCC treatment either as a single agent or jointly with sorafenib.
Bispecific antibody (bsAb)
The BioNTech team fabricated 6PHU3, a T-cell-engaging bispecific single-chain molecule [bi-(scFv)2] with anti-CD3 or anti-Claudin 6 specificities. It is targeted to induce T cells to kill Claudin 6+ tumor cells by triggering T cells and specifying its pharmacodynamic properties.
The in vivo efficacy experiments affirmed that the highly selective targeting of 6PHU3 is efficient. 6PHU3 is probably an efficient treatment for Claudin 6+ solid tumors.
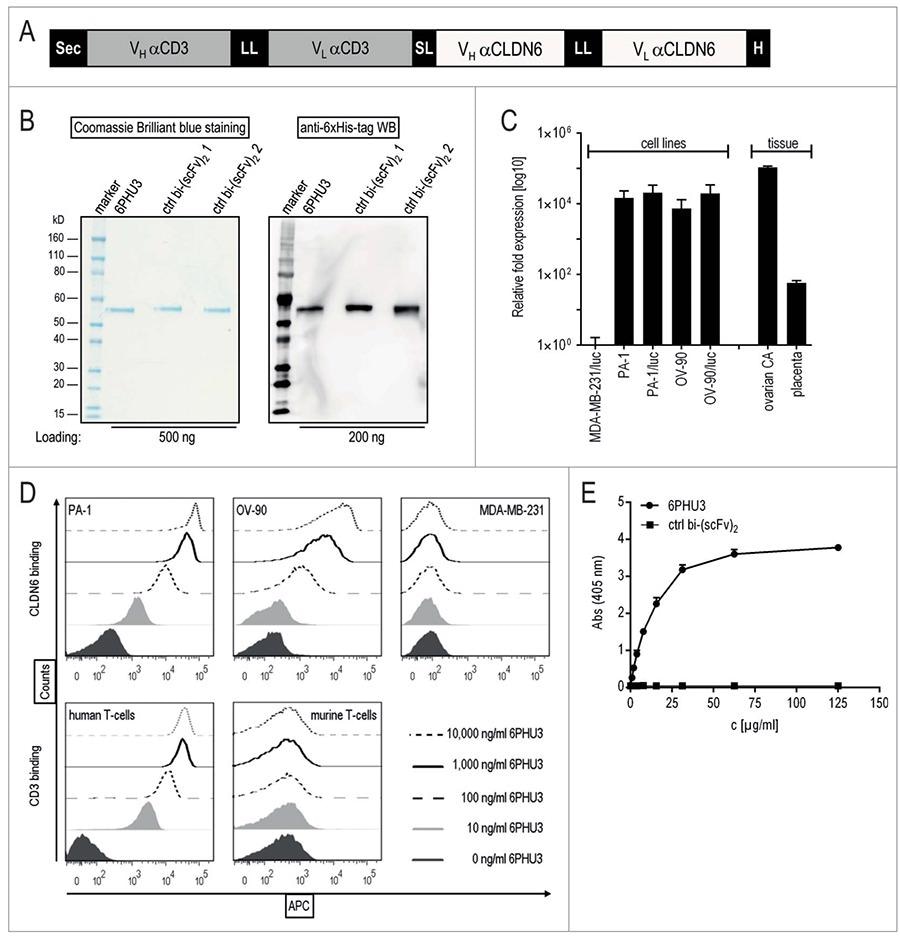
6PHU3 binds to CLDN6 and CD3 selectively. Image from reference
CAR-T cell therapy
The accuracy specificity, high sensitivity, and strength of CAR to Claudin 6, a surface molecule, make Claudin 6 a perfect target for CAR-T cell therapy for solid cancers. Besides anti-Claudin 6/CD3 bsAb (BNT142), BioNTech’BNT211 is also known as a CAR-T cell therapy targeting Claudin 6.
It is utilized in the early clinical development for the treatment of solid tumors and is presently in Clinical Phase I/II.
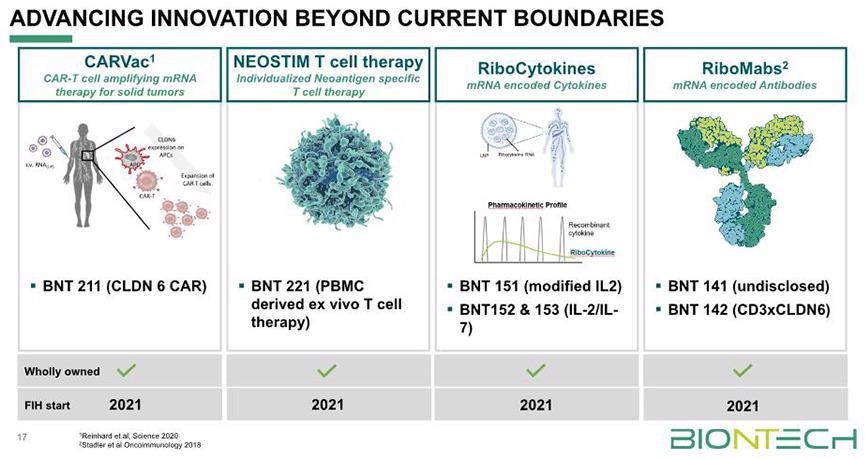
Partial pipeline of BioNTech. Image from reference
Summary
Claudin 6 is explicitly silenced in healthy adult tissues, but abnormally activated and expressed in a variety of solid tumors with high medical needs. As a result, Claudin 6 is thought to be a promising cancer immunotherapy target.
The development of different drug types that target Claudin 6, such as mAbs, ADCs, bsAbs and CAR-T cell therapy, has given solid tumor immunotherapy new strategies and hope.
ACROBiosystems has been successful in developing HEK293 expressed full-length Claudin 6-VLP protein (Met 1-Val 220) through “FLAG” to aid drugs and therapies R&D targeting Claudin 6.
Product list
Source: ACROBiosystems
| Molecule |
Cat. No. |
Product Description |
Application |
| Claudin-6 |
CL6-HF2G5 |
Human Claudin-6 / CLDN6 Full Length Protein-VLP (HEK293)New |
Immunization
ELISA
SPR/BLI
Cell-based Assay
… |
| VLP |
VLP-H52C5 |
Virus-Like Particle (VLP) isotype control VLP |
Isotype Control |
Product features
- Full-length Claudin 6 protein available with native and entire conformation
- Ideal for immunization/ELISA/SPR/BLI/cell experiment etc.
- Higher immunogenicity as a result of inherent characteristics of VLP
- High biological activity is verified by binding to antibodies
- 100 to 300 nm in size and high identity confirmed by DLS and could be utilized as an optimal target for phage display and dendritic cells
Verification data
Full-length Claudin 6-VLP protein (Cat. No. CL6-HF2G5)
High bioactivity verified by ELISA
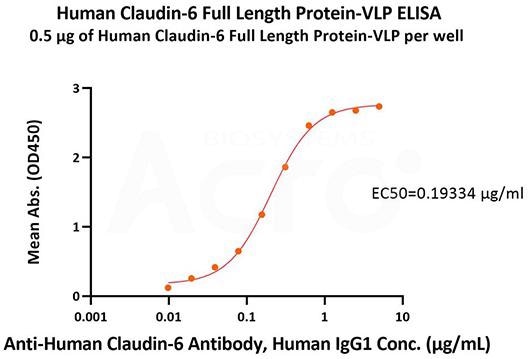
Immobilized Human Claudin-6 Full Length Protein-VLP (Cat. No. CL6-HF2G5) at 5 μg/mL (100 μL/well) can attach to Anti-Human Claudin-6 Antibody, Human IgG1 with a linear range of 0.01–0.625 μg/mL (QC tested). Image Credit: ACROBiosystems
High identity verified by DLS
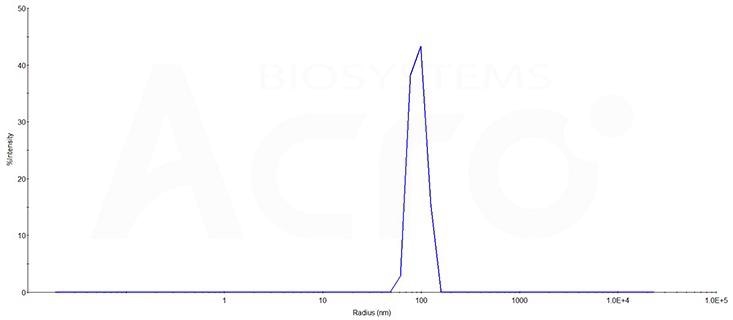
The mean peak Radius of Claudin 6-VLP (Cat. No. CL6-HF2G5) is 80–100 nm with more than 95% intensity as determined by dynamic light scattering (DLS). Image Credit: ACROBiosystems
References
- Kong F E., et al. Targeting tumor lineage plasticity in hepatocellular carcinoma using an anti-CLAUDIN 6 antibody-drug conjugate[J]. Science Translational Medicine, 2021, 13(579), doi: 10.1126/scitranslmed.abb6282
- Junko Matsuzaki, Shashikant Lele, Kunle Odunsi&Takemasa TsujiIdentification of Claudin 6-specific HLA class I- and HLA class II-restricted T cellreceptors for cellular immunotherapy in ovarian cancer, OncoImmunology, 2022, 11:1, 2020983, https://pubmed.ncbi.nlm.nih.gov/35003898/
- https://www.pharmacodia.com/
- https://investors.biontech.de/node/9121/html
- Christiane R. Stadler, Hayat Bähr-Mahmud, Laura M. Plum, Kathrin,Schmoldt, Anne C. Kölsch, Özlem Türeci &UgurSahin (2016) Characterization of the firstin-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solidtumors expressing the oncofetal protein Claudin 6, OncoImmunology, 5:3, e1091555, https://pubmed.ncbi.nlm.nih.gov/27141353/
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.