At the 20th European Crohn's and Colitis Organization (ECCO) Congress in February 2025, Sanofi and Teva Pharmaceuticals presented detailed results from the Phase 2b RELIEVE UCCD clinical trial for Duvakitug.
Duvakitug is their jointly developed inflammatory bowel disease (IBD) monoclonal antibody treatment.
The trial results showed that patients with ulcerative colitis (UC) and Crohn's disease (CD) who received Duvakitug achieved remission by week 14, demonstrating the drug’s potential to become a "Best-in-Class" medication.
A Phase III clinical trial is expected to begin in the second half of 2025, moving this breakthrough treatment closer to clinical application.
The "Immortal" cancer: Enhancing clinical remission rates as a key strategy in IBD treatment
There are two primary types of IBD: UC and CD, both characterized by chronic gastrointestinal inflammation.
Patients suffering from these diseases frequently report abdominal pain, diarrhea, and rectal bleeding as symptoms.
Prolonged inflammation can cause fibrosis, intestinal strictures, and blockages, which can sometimes require surgical intervention.
Studies have found Tumor Necrosis Factor-like Ligand 1A (TL1A) binding to its receptor DR3 increases problematic immunological responses and accelerates IBD-related fibrosis.
By targeting TL1A, excess immunological activity can be reduced, delaying disease progression and enhancing patient outcomes.
As IBD is incurable, current treatment techniques center on inducing remission, preventing relapse, and improving patient quality of life.
Duvakitug, a human IgG1-λ2 monoclonal antibody targeting TL1A, has shown promising efficacy in its most recent Phase 2b clinical trial.
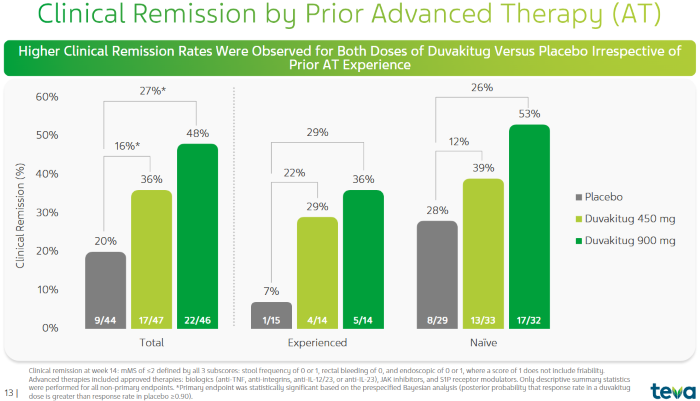
Comparison of Clinical Remission Rates of Duvakitug in UC Patients with and without Prior Advanced Therapy (Prior AT)2. Image Credit: ACROBiosystems
The figure above shows a 36% remission rate for UC patients in the 450 mg dose group (modified Mayo score, mMS) on average across both naïve and experienced patients. However, an even higher result was seen in the 900 mg dose group, which had a remission rate of 48%. This compares to the placebo group's 20% remission rate.
For patients who had previously received advanced therapy, the clinical remission rates in the 450 mg and 900 mg dosage groups were slightly lower, at 29% and 36%, respectively, compared to the placebo group's 7%.
Among patients without prior advanced therapy, the 450 mg and 900 mg dosage groups had remission rates of 39% and 53%, respectively, compared to 28% in the placebo group.1
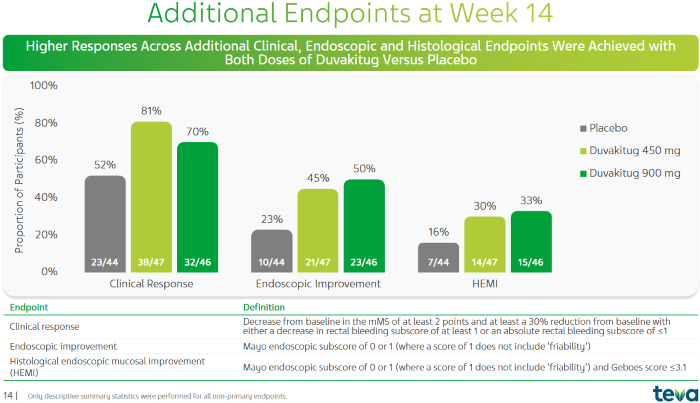
Clinical Response Rate, Endoscopic Improvement (MES), and Histologic-Endoscopic Mucosal Improvement (HEMI) of Duvakitug in the Treatment of UC Patients2. Image Credit: ACROBiosystems
Significant improvements were seen in UC patients, with both the 450 mg and 900 mg dosage groups demonstrating improved clinical response rate, endoscopic improvement (MES), and histologic-endoscopic mucosal improvement (HEMI).
The clinical response rates were 81% and 70%, respectively, substantially higher than the placebo group's 52%. The MES rates were 45% and 50%, respectively, vs 23% in the placebo group.
There was some HEMI improvement, with the two dosing groups reaching 30% and 33% respectively, compared to 16% in the placebo group.1
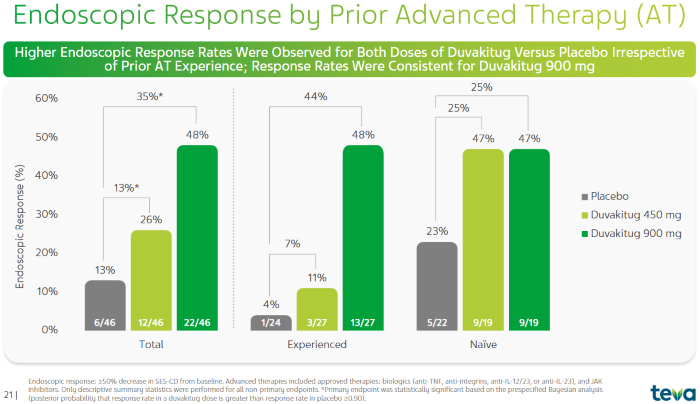
Comparison of Clinical Remission Rates of Duvakitug in CD Patients with and without Prior Advanced Therapy (Prior AT)2. Image Credit: ACROBiosystems
Duvakitug was also effective in CD patients. Overall, 26% of patients in the 450 mg dose group achieved clinical remission. Again, a higher result was seen in the 900 mg group with a 48% remission rate, much greater than the placebo group's 13%.
For patients who had previously received advanced therapy treatment, the 450 mg and 900 mg dosage groups had remission rates of 11% and 48%, respectively, compared to 4% in the placebo group.
Among patients who had not previously had advanced therapy, the 450 mg and 900 mg dosage groups both had remission rates of 47%, which was higher than the placebo group's 23%.1
Despite its "Best-in-Class" potential, the competition remains fierce
Current estimates expect the global autoimmune disease drug market to grow to $140 billion by 2028, according to Evaluate Pharma. Within that estimate, IBD drugs are expected to account for $28 billion, or 20% of the market.
Two anti-TL1A medicines are ahead of Duvakitug in clinical development: Merck's PRA023 and RVT-3101, a collaboration between Roivant and Pfizer.
PRA023
PRA023 has shown high efficacy in clinical trials for UC and CD patients.
In the Phase 2 clinical research trial ARTEMIS-UC, which focused on treating moderate to severe UC, UC patients achieved a clinical remission rate of 26.5%, which was significantly greater than the placebo group's 1.5%.
In addition, 36.8% of patients met the secondary goal of endoscopic improvement, against just 6% in the placebo group. The overall clinical response rate increased by about 44% (from 22.4% to 66.2%).
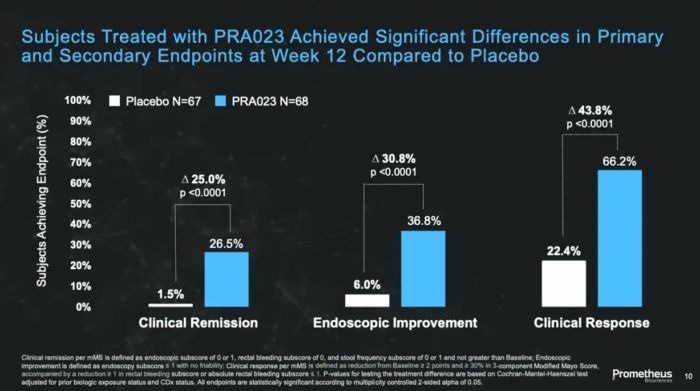
Phase 2 Clinical Trial Data of PRA023 for UC Patients - ARTEMIS-UC Study3. Image Credit: ACROBiosystems
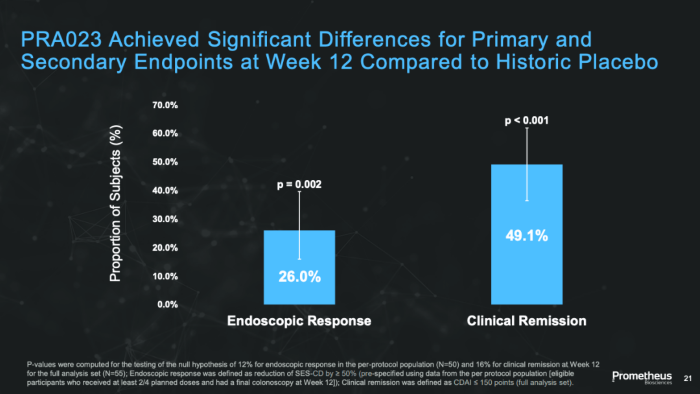
Phase 2a Clinical Trial Data of PRA023 for CD Patients - APOLO-CD Study3. Image Credit: ACROBiosystems
RVT-3101
In its Phase 2 clinical trial for IBD, RVT-3101 achieved a clinical remission rate of 31% in UC patients, which further improved to 40% in biomarker-positive patients, with an endoscopic remission rate of 56%.
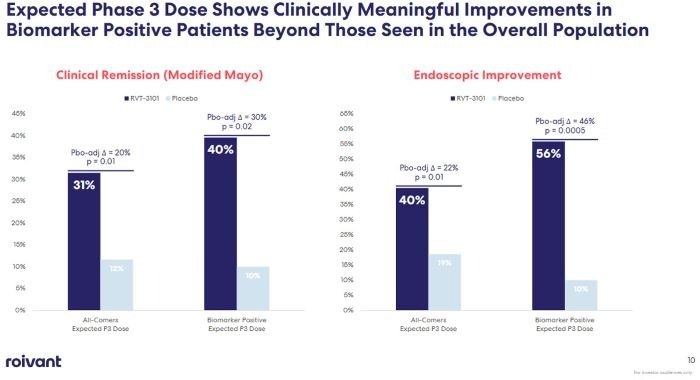
Phase 2 Clinical Data of RVT-3101 for UC4. Image Credit: ACROBiosystems
However, Duvakitug efficacy outperformed both PRA023 and RVT-3101 in numerous important clinical endpoints, showing consistent effects in patients with differing treatment histories. However, there is still room for improvement.
In its current stage, Duvakitug requires medication every two weeks, whereas both competitors provide a more convenient four-week dosing schedule.
To compete with PRA023 and RVT-3101, Sanofi and Teva pharmaceuticals need to expedite Phase III trials and adjust dose regimens to increase patient adherence.
Overall, the emergence of TL1A-targeting treatments present new opportunities for IBD treatment, accelerating progress in the field. The competitivity of the autoimmune disease therapy market will ultimately benefit IBD patients, improving their lived experience.
Comprehensive TL1A-targeted drug development solutions
ACROBiosystems has introduced several high-quality instruments to meet biologic drug development needs for IBD, from the molecular to the cellular level:
Natural trimer validation of TL1A, DR3, and DcR3 recombinant proteins
The SEC-MALS verified the native trimer structure of the TL1A protein. Both TL1A and DR3 proteins are expressed in human cells, spanning a wide range of tags with good purity, activity, and consistency from batch to batch.
These are appropriate for use in immunoassays, antibody screening, and candidate drug functional validation.
Human DR3 (TL1A receptor) (Luc) Jurkat reporter cell development service
This Cell Development Service has been verified through receptor expression and functional activity.
It can be used in research on TL1A agonist drug signaling pathways, cell-based drug activity assays and screening, and drug CMC quality control and release.
Development services for HEK293/human TL1A stable cell line, HEK293/membrane-bound human TL1A stable cell line, and Raji/membrane-bound human TL1A stable cell line
The antigen is stably expressed on the host cell membrane throughout time, making it useful for applications such as antibody drug activity screening (cell-level binding and blocking) and CAR molecular cytotoxicity evaluation.
Membrane-bound cell lines closely resemble the physiological interactions of TL1A and its receptors (such as DR3) on the cell membrane. They are suitable for investigating membrane-bound signaling cascades and receptor interactions in drug development.
These cell lines are appropriate for anti-TL1A drug screening, receptor function studies, signal transduction analysis, and high-throughput drug screening.
TL1A [biotinylated]: DR3 inhibitor screening ELISA kit
This kit is specifically designed for detecting and screening inhibitors of the TL1A-DR3 signaling pathway. It efficiently assesses the inhibitory effect of candidate compounds on TL1A binding to its receptor DR3.
With high sensitivity and specificity, the DR3 Inhibitor Screening ELISA kit is ideal for high-throughput screening in drug development, allowing researchers to quickly find promising TL1A-targeted inhibitors and providing strong support for biologic therapy in conditions like inflammatory bowel disease.
Human TL1A-DR3 inhibition kit
This kit uses Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) technology to quantify the interaction between TL1A and DR3.
Its excellent sensitivity and low background interference make it ideal for screening putative TL1A-DR3 signaling pathway inhibitors.
ACROBiosystems conducts batch-by-batch quality control on all products, evaluating qualities such as purity and binding activity, and offers free protocols containing experimental parameters and processes created by the research team to help minimize R&D time.
References
- Sanofi. (2025). Press Release: ECCO 2025: new duvakitug data reinforce best-in-class potential in ulcerative colitis and Crohn’s disease. (online) Available at: https://www.sanofi.com/en/media-room/press-releases/2025/2025-02-22-07-30-00-3030764.
- Teva Pharmaceutical Industries Ltd. (2019). Teva Pharmaceutical Industries Ltd. - Duvakitug (Anti-TL1A) Phase 2b additional data presentation after ECCO – Conference call. (online) Available at: https://ir.tevapharm.com/Events-and-Presentations/events-and-presentations/event-details/2025/Teva-call/default.aspx (Accessed 24 Jun. 2025).
- SEC. (2022). EX-99.1. (online) Available at: https://www.sec.gov/Archives/edgar/data/1718852/000119312522299773/d420864dex991.htm (Accessed 24 Jun. 2025).
- LARVOL. (2023). RVT-3101: “Statistically significant and clinically meaningful efficacy results observed at every dose tested and in both overall and biomarker positive populations“; Ulcerative colitis. (online) Available at: https://delta.larvol.com/NewsItem/Default.aspx?NewsItemID=220ba66f-7e9a-4955-a0ae-03c63c34529a&ts=638763024105251773 (Accessed 24 Jun. 2025).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.