Alzheimer's disease (AD) is a major global public health concern. Although monoclonal antibody medicines targeting beta-amyloid (Aβ) have achieved approval, there is still an urgent and unmet need for more effective and precise therapeutic techniques.
Professor Jeffrey Cummings and his colleagues' new thorough assessment: “Alzheimer’s Disease Drug Development Pipeline: 2025” sheds light on the developing AD medication research landscape.
This analysis distills their findings into three essential dimensions: pipeline expansion, target diversification, and technological innovation. This results in a strategic review of the most recent trends and breakthroughs in AD research and development.

Image Credit: ACROBiosystems
Pipeline expansion: 138 drugs active in 182 clinical trials
As of January 1, 2025, 138 AD candidate medications were being tested in 182 clinical trials worldwide, a large increase over the previous year (127 drugs, 164 trials).
The pipeline now consists of 48 Phase III (31 medications), 86 Phase II (75 drugs), and 48 Phase I (45 drugs). Notably, the number of Phase I trials has increased by 85% since 2024 (from 26 to 48), demonstrating the industry's rising emphasis on innovative mechanisms of action.
Disease-targeting therapeutics (DTTs) dominate the pipeline, accounting for 74 % (60 small molecules and 42 biologics).
In addition, 14 % of therapies (19 in total) seek to improve cognitive function by altering the cholinergic system or glutamate receptors to slow memory deterioration. Another 11 % (15 medicines) treat neuropsychiatric symptoms (NPS) such as agitation, psychosis, and apathy.
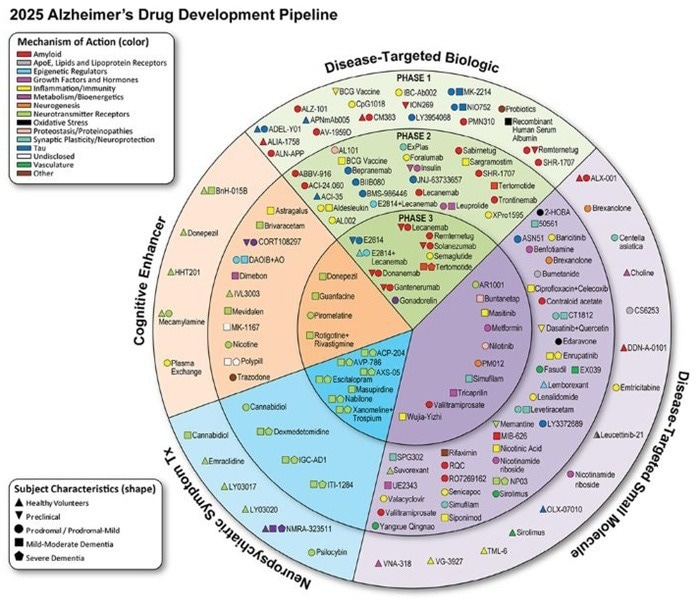
As of January 1, 2025, drugs in clinical trials for AD treatment registered on clinicaltrials.gov. Image Credit: ACROBiosystems
Target diversification: Moving beyond Aβ and Tau
The Common Alzheimer's Disease Research Ontology (CADRO) categorizes pharmacological targets and mechanisms into 15 AD-related pathology groups. At least one drug is now under development for each category, demonstrating a shift in Alzheimer's drug development toward multi-mechanistic and multidimensional approaches.
AD medication development continues to focus on classic pathological pathways such Aβ deposition and Tau hyperphosphorylation, accounting for 18 % and 11 % of current investigational medicines, respectively.
Neurotransmitter receptors (22 %), neuroinflammation/immune response (17 %), synaptic plasticity/neuroprotection (6 %), energy metabolism (6 %), growth factor, lipid metabolism, protein homeostasis, vascular function, and circadian rhythm are emerging as hotspots in Alzheimer's disease research.
More cutting-edge approaches, including medicines targeting the gut-brain axis and epigenetic control, reflect the growing understanding of AD's complex etiology.
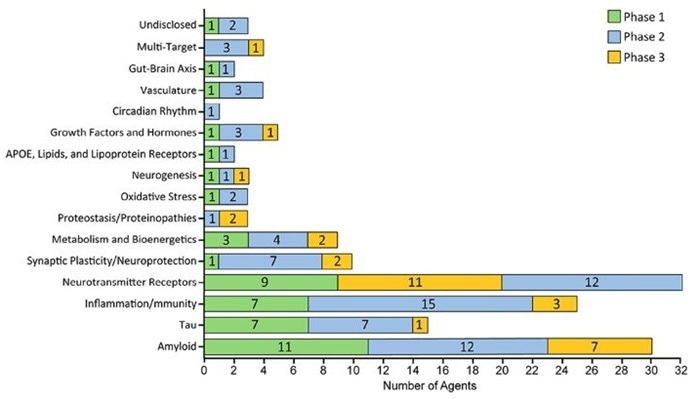
Number of AD drug candidates by mechanism of action across different stages of development. Image Credit: ACROBiosystems
Technological innovation: Advancements in efficiency driven by biomarkers, combination therapies, and drug repurposing
Compared to previous AD clinical trial designs that depend predominantly on symptom scores as the primary endpoints, modern AD medication clinical research has made substantial advances in the use of biomarkers.
In 182 AD clinical studies, 57 % employed biomarkers as inclusion or exclusion criteria, while 27 % used them as primary outcomes. The most often used imaging biomarkers are MRI and PET (Aβ).
Fluid biomarkers, such as p-tau217 and the Aβ42/40 ratio, are being used in clinical studies for screening and evaluating efficacy. Furthermore, researchers are increasingly interested in combination therapy.
Currently, 20 clinical trials are evaluating various combination strategies, including synergistic pharmacodynamic combinations—such as dasatinib and quercetin, which target neuroinflammation in the treatment of Alzheimer's disease—and pharmacokinetically optimized combinations, such as dextromethorphan combined with a CYP2D6 inhibitor to improve efficacy by slowing hepatic metabolism.
Conclusion
The AD drug development pipeline is marked by a trend of increased scale, target diversification, and technological advancement. Recent research has expanded beyond traditional Aβ and Tau-targeting techniques to address inflammation, metabolism, and neurotransmission.
The increased use of biomarkers and the development of combination therapy techniques are driving clinical research toward better precision and efficiency. The expansion of treatment targets and technologies ushers in an exciting new age in Alzheimer's medication research, paving the way for more individualized and effective patient care.
ACROBiosystems’ solutions: Driving progress in AD research
Aneuro, ACROBiosystems' neuroscience-focused brand, provides cutting-edge solutions such as target proteins, pre-formed fibrils (PFFs), brain organoids, and p-tau antibodies to speed Alzheimer's disease research and development.

Image Credit: ACROBiosystems
Image Credit: ACROBiosystems
Reference
- Cummings, J.L., et al. (2025). Alzheimer’s disease drug development pipeline: 2025. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 11(2). https://doi.org/10.1002/trc2.70098.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.