In recent years, major breakthroughs have been made in the development and treatment of small cell lung cancer (SCLC). Research has increasingly focused on the development of Delta-like ligand 3 (DLL3)-targeted bispecific antibodies and antibody-drug conjugates (ADCs).
DLL3 has emerged as a promising therapeutic target because of its high, specific expression on the surface of SCLC and neuroendocrine tumour cells, while showing minimal presence in healthy tissue.
Pharmaceutical companies, including AbbVie and Amgen, are driving clinical research on DLL3-targeted therapies, and several drug candidates are already in advanced stages of development.
Innovent licenses DLL3-ADC to Roche for more than $1 billion
On 2 January 2025, Innovent Biologics announced an exclusive global collaboration with Roche, licensing the worldwide rights to its DLL3-ADC candidate, IBI3009.
Under the deal, Innovent received an upfront payment of $80 million, with the potential to earn up to $1 billion in development and commercialisation milestone payments. It also received tiered royalties based on global annual net sales.
IBI3009 is developed using Innovent's next-generation camptothecin-based ADC platform NT3. Image Credit: ACROBiosystems
IBI3009 has already obtained IND approvals in China, Australia, and the United States. Clinical studies are actively underway, with the first patient successfully enrolled.
This highly anticipated DLL3-ADC candidate was not developed using the previously utilized Lonza Synaffix platform. Instead, it was designed using NT3, Innovent’s proprietary next-generation camptothecin-based ADC platform.
NT3 leverages the novel topoisomerase I inhibitor (TOPO1i) Exatecan as the payload, using powerful structural optimization and advanced conjugation technology to enhance ADC therapeutics’ efficacy and safety.
Research has shown that Exatecan-based ADCs considerably improve antitumor activity and reduce off-target toxicity compared to conventional payloads like MMAE/MMAF. These findings have been especially prominent when treating highly heterogeneous tumors such as those present in lung, gastric, and breast cancer.
IBI3009’s higher DAR and improved potential tumor-killing efficiency mean it represents a promising new treatment option for patients with refractory tumors.
Innovent’s NT3 platform enhances ADC drug advantages
A PCT update from October 3, 2024, revealed that Innovent Biologics had disclosed patent WO2024208359. This new patent covered NT3, the company’s independently developed next-generation camptothecin ADC platform, and its applications.
The patent in question outlined the development of a novel HER2-ADC drug that leverages camptothecin derivatives as cytotoxic payloads, targeting specific cancer cell antigens and enabling the delivery of camptothecin derivatives directly to cancer cells. This approach helps to improve therapeutic efficacy while limiting systemic toxicity.
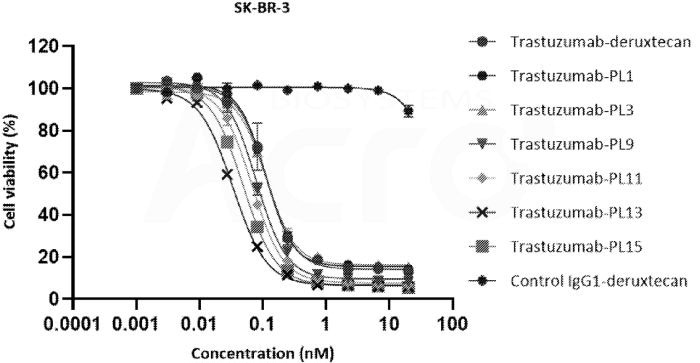
Innovent's HER2 camptothecin-based ADC demonstrates superior in vitro activity compared to Daiichi Sankyo's DS-8201. Image Credit: ACROBiosystems
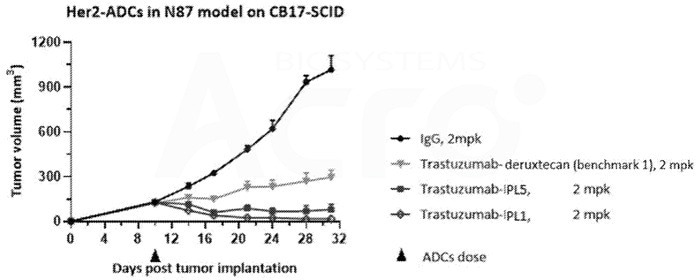
In the N78 model, the HER2 camptothecin-based ADC exhibits superior in vivo activity compared to Daiichi Sankyo's DS-8201. Image Credit: ACROBiosystems
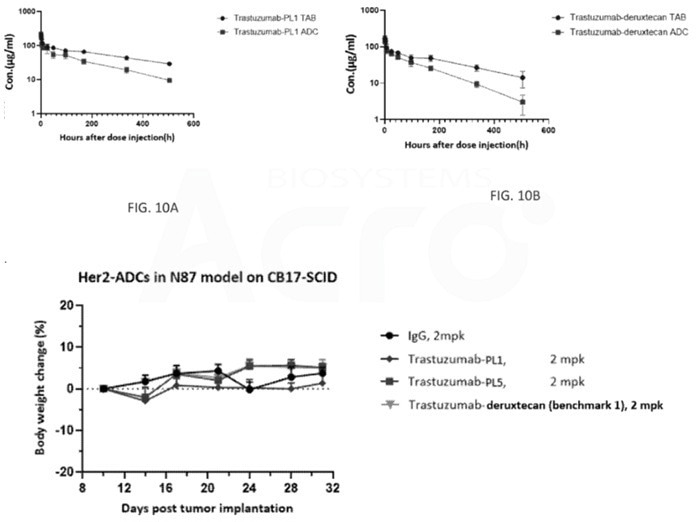
The pharmacokinetics and toxicity risks of the PL1 constructed HER2 camptothecin ADC are similarly low compared to DS-8201. Image Credit: ACROBiosystems
Innovent Biologics has developed a total of twenty camptothecin derivative toxins by leveraging its NT3 self-toxin platform. The PL1 toxin uses traditional cysteine conjugation with a DAR value of eight, while the HER2 camptothecin ADC created by Innovent has been shown to improve in vitro activity versus Daiichi Sankyo’s DS-8201.
The PL1-created ADC also shows significantly better anti-tumor efficacy in the N87 model than DS-8201. Both the PL1-based ADC and DS-8201 demonstrate low toxicity risks and similar pharmacokinetic profiles.
The landmark partnership between Roche and Innovent was created at the start of 2025 to highlight Roche's multi-path SCLC drug development strategy.
Roche's RG6524 DLL3-targeted tri-specific antibody functions as an upgraded version of the bispecific antibody. While both target DLL3, RG6524 and DLL3-ADC IBI3009 differ in terms of their specific mechanisms of action.
It is possible to combine RG6524 and IBI3009 with PD-L1 antibodies to form two complementary treatment approaches.
DLL3 is also a key therapeutic target in SCLC. AbbVie's DLL3-ADC had failed previously, but this was primarily due to limited understanding of the toxin and ADC, which has since expanded and improved.
Current clinical data suggests that TOPO1i toxins may offer enhanced drug development potential due to their low toxicity and reduced bystander effects.
While Roche's RG6524 trispecific antibody displays improved efficacy over bispecific antibodies in preclinical studies, TCE antibodies’ higher efficacy can lead to greater toxicity.
IBI3009 remains a viable alternative even if RG6524 fails in clinical trials, ensuring Roche's ongoing development and improved positioning in DLL3-targeted therapies.
ACROBiosystems’ contribution
ACROBiosystems has developed a portfolio of products to meet the needs of DLL3-targeted small cell lung cancer ADCs and antibody drug developers.
High-quality DLL3 recombinant proteins
These proteins are expressed in human cells and verified using ELISA, SEC-MALS, SPR, and BLI. Each offers high activity, purity, and exceptional batch-to-batch consistency, making them well-suited for antibody screening, immunology, and candidate drug function validation.
High-quality DLL3 overexpressing cell lines (CHO, HEK293)
This antigen is stably expressed on the surface of host cell membranes, making it ideally suited to both antibody drug activity screening (cell-level binding and blocking) and the evaluation of CAR molecule cytotoxicity.
Summary
ACROBiosystems confirms key properties such as purity and binding activity by performing batch-by-batch quality control on all products. The company’s research team also provides a range of free protocols with experimental parameters and procedures to help its customers save vital R&D time.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.