Statins have been the primary therapy for reducing low-density lipoprotein cholesterol (LDL-C) and preventing atherosclerotic cardiovascular disease (ASCVD) since the 1980s. However, many patients taking statins still struggle to achieve optimal cholesterol levels.
Recently, innovative therapies have emerged based on oligonucleotides and gene editing, offering broader benefits, longer-lasting effects, and the possibility of a one-time, permanent reduction in cholesterol levels.
Lowering LDL-C levels: Key to preventing ASCVD risk
As living standards have improved and society has changed, modern lifestyles have introduced significant health challenges. The accompanying ways of modern life–increased work stress, sedentary habits, and poor dietary choices–can be major triggers of cardiovascular diseases. The rising incidence of atherosclerosis due to hyperlipidemia poses a massive threat to human health.
As a consequence of this, research into maintaining cardiovascular health and exploring new therapeutic targets to further reduce residual cardiovascular risk is essential in developing lipid-lowering therapies.
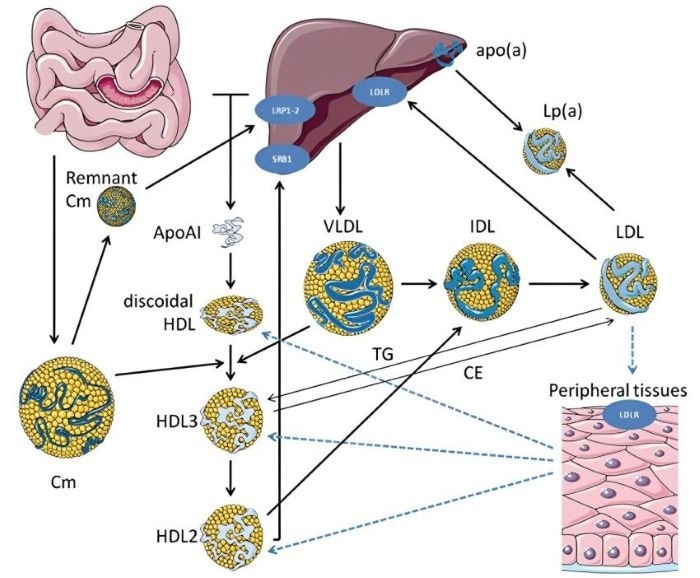
Lipid Metabolism Pathway 1. Image Credit: ACROBiosystems
Atherosclerosis is a metabolic disease caused by hyperlipidemia. Under normal conditions, intact endothelial cells allow efficient transport and metabolism of lipids via lipoprotein particles. However, damage to the vascular endothelium causes the accumulation of cholesterol-rich low-density lipoprotein (LDL) in vessel walls and can lead to atherosclerosis.
Local inflammation and oxidative stress increase as macrophages engulf LDL, causing further atherosclerotic plaque formation and worsening vascular health.
Lipid profile markers commonly include total cholesterol, triglycerides, LDL-C, and high-density lipoprotein cholesterol (HDL-C). However the actual composition of lipids is more complex, with a range of different components present, such as lipoprotein (a) (Lp(a)), free fatty acids, and phospholipids.
These lipids bind to apolipoproteins to form various lipoprotein particles, allowing them to maintain their solubility in the bloodstream and be transported throughout the body to support cellular metabolism. Differing sizes and density of lipoproteins affect their ability to infiltrate arterial walls and contribute to plaque formation.
LDL is the primary carrier of cholesterol in the blood, accounting for approximately 70 % of total blood cholesterol levels. As a result, lowering LDL-C levels is a central strategy in reducing ASCVD risk.
Innovative therapies leading the new direction in lipid-lowering
Although statins are effective at lowering LDL-C and preventing cardiovascular diseases, they have several limitations.
Firstly, statin effectiveness varies among individuals, and some patients do not achieve optimal cholesterol levels. There are also some side effects to statins, including mild muscle pains and weaknesses, liver function abnormalities. An increased risk of diabetes can also affect the adherence of statins.
Statins show limited effects on other lipid components and cannot fully eliminate all cardiovascular diseases. Genetic factors also influence the efficacy of statins, with varying outcomes from person to person.
Emerging oligonucleotides and gene editing therapies offer greater precision, long-term efficacy, and improved adherence in comparison to traditional statin therapies. This is especially true for statin-intolerant or patients who are unresponsive to statins.
RNA interference (RNAi) therapies: Strong adherence and broad benefits
Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) promotes the degradation of LDL receptors (LDL-R), increasing LDL-C levels and leading to a higher risk of atherosclerosis.
Novartis' RNAi therapy, Leqvio (Inclisiran), uses RNA interference to reduce PCSK9 levels by binding to the mRNA that encodes to the protein. This prevents the liver from producing PCSK9, which in turn promotes the LDL-R recycling and effectively clears LDL-C. Leqvio has been approved by the FDA for treating adult patients with primary hyperlipidemia, including those with heterozygous familial hypercholesterolemia (HeFH).
Leqvio requires only two injections per year, compared to other PCSK9 inhibitors, and works in tandem with statins to improve patient outcomes. In 2023, Leqvio received FDA approval for expanded indication to treat patients with elevated LDL-C and increased cardiovascular risk.
In August 2024, Novartis announced that Leqvio had shown successful reduction of elevated LDL-C levels in patients with medium and low-risk ASCVD in Phase 3 V-MONO trials. These results support Legvio’s broader application potential as a cardiovascular disease prevention therapy.2
Zodasiran, Arrowhead Pharmaceuticals' targeted angiopoietin-like 3 (ANGPTL3) RNAi therapy has recently entered Phase 3 clinical trials. Phase 2 trial results of the drug, published in May 2024, showed that in patients administered with the highest dose of Zodasiran, triglyceride levels were reduced by 63 % and residual cholesterol levels were reduced by 82 % after 24 weeks.3
Development of Lp(a) antisense oligonucleotide lipid-lowering therapies
Lp(a) is an independent genetic risk factor for cardiovascular diseases, affecting about 20 % of the global population. As it is genetic, levels of Lp(a) cannot be altered by lifestyle changes. Currently there is no effective therapy specifically targets lowering Lp(a) levels, despite attempts to regulate it with drugs like niacin.
Research shows that PCSK9 monoclonal antibodies can lower Lp(a), while also reducing LDL-C levels. As a result, these antibodies can provide significant cardiovascular protection for patients with high baseline Lp(a) levels. There are several Lp(a)-targeted antisense oligonucleotide therapies in clinical development, including Novartis' Pelacarsen, Amgen's Olpasiran, Ionis' Zerlasiran, and Eli Lilly's Lepodisiran.
These trials aim to assess the efficacy and safety of PCSK9 monoclonal antibodies in reducing Lp(a) levels.4 So far, Amgen's Olpasiran in the OCEAN-DOSE trial showed that even after one year of discontinuation, patients' Lp(a) levels remained low, with over 98 % of them reducing to standard values of 125 nmol/L or lower.
Eli Lilly's RNAi therapy, Lepodisiran, is also in development and has entered Phase 3 trials. Early data from these studies indicate that a single treatment can sustain an almost one-year reduction in Lp(a) levels, demonstrating that it’s efficacy is durable.5
Base editing therapies offer potential for one-time lipid-lowering treatment
Building on these oligonucleotide therapies that have shown reduced treatment frequency, gene editing therapies offer a longer-lasting solution which show potential as a permanent way to reduce Lp(a) levels.
Verve Therapeutics has developed VERVE-102, a base editing therapy that targets the PCSK9 gene, permanently inactivating it and effectively lowering LDL-C levels.
This therapy uses Verve's proprietary GalNAc-modified lipid nanoparticle (LNP) delivery technology for specific liver targeting and improved tolerability. VERVE-102 is currently undergoing Phase 1b clinical trials and has been highlighted as one of the top ten clinical trials to watch in 2025.6
Another base editing therapy from Verve, VERVE-201, is also in Phase 1b trials, using the GalNAc-LNP platform to deliver a base editor targeting the ANGPTL3 gene, a key regulator of cholesterol and triglyceride levels. It aims to permanently disrupt ANGPTL3 protein production, lowering LDL-C and residual cholesterol.
Compared to PCSK9-targeted editing therapies, ANGPTL3-targeted therapies benefit from lipid nanoparticles with GalNAc ligands, allowing uptake in patients with homozygous familial hypercholesterolemia (HoFH), who lack LDL-R, to also benefit from the therapy by facilitating drug uptake into the liverthus expanding the patient population.7
High-quality tools for lipid metabolism research and drug development
ACROBiosystems provides a comprehensive suite of tools to support the development of oligonucleotide, gene-editing, and antibody-based therapies targeting lipid metabolism. These include:
- PCSK9 and ANGPTL3 recombinant proteins: Expressed in human-derived HEK293 cells and validated by SDS-PAGE, SEC-MALS, ELISA, and SPR, these proteins have high purity, activity, and consistency. They are suitable for immunization, antibody screening, and validation of candidate drug functionality.
- PCSK9 [Biotinylated]: The LDL R Inhibitor Screening ELISA Assay Pair uses unique biotinylated proteins to develop high-quality inhibitor screening reagents. It comes with applicable methodologies for inhibitor screening and quality control.
- Various targeted drug development tools for hyperlipidemia: These tools include ASGR1, THRA, THRB, LDL-R, APOA-II, APOE, APOH, and LPL, fully supporting drug development research.
All ACROBiosystems products undergo rigorous batch-to-batch quality control, ensuring verified purity and binding activity. They also provide free protocols to streamline drug development efforts.
References
- Badimon, L. and Chiva-Blanch, G. (2019). Lipid Metabolism in Dyslipidemia and Familial Hypercholesterolemia. The Molecular Nutrition of Fats, pp.307–322. https://doi.org/10.1016/b978-0-12-811297-7.00024-x.
- Novartis. (2024). Novartis Pipeline | Novartis. (online) Available at: https://www.novartis.com/research-development/novartis-pipeline?search_api_fulltext=Leqvio (Accessed 26 Jun. 2025).
- Arrowhead Pharmaceuticals, Inc. Pipeline. (online) Available at: https://arrowheadpharma.com/pipeline/.
- Amgen. (2023). AMGEN PRESENTS LATE-BREAKING PHASE 2 OLPASIRAN DATA AT ESC 2023. (online) Available at: https://www.amgen.com/newsroom/press-releases/2023/08/amgen-presents-late-breaking-phase-2-olpasiran-data-at-esc-2023.
- Nissen, S.E., et al. (2023). Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA, (online) p.e2321835. https://doi.org/10.1001/jama.2023.21835.
- Verve Therapeutics. Verve 101 & 102 | Verve Therapeutics. (online) Available at: https://www.vervetx.com/our-programs/verve-101-102.
- Vervetx. (2025). Verve 201 | Verve Therapeutics. (online) Available at: https://www.vervetx.com/our-programs/verve-201.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.