Chimeric antigen receptor T cell therapy (CAR-T cell therapy) is a new kind of immunotherapy with encouraging results in the treatment of hematological malignancies. At present, CD19 is the most commonly used target in CAR-T cell therapy.
CD19 CAR T cell therapy is approved for the safe and efficient treatment of CLL, B-ALL and B cell lymphoma.
As of yet, there are only four FDA-approved CD19 targeted CAR-T cell therapy drugs — Yescarta, Kymriah, Breyanzi and Tecartus. It is vital to analyze CAR expression in the production of CAR-T cells and to supervise the persistence after administration.
Two robust tools for identifying CD19 CAR expression at the quality control and clinical trial stages are discussed below.
Image Credit: Britten, Oliver, et al. 2019
Introduction
CD19 protein can bind specifically to the antigen-binding domains of its CARs and analyze CAR functionality which makes CD19 an ideal reagent for CD19 CAR identification in quality control. Additionally, FMC63 is an IgG2a mouse monoclonal antibody targeting CD19.
The majority of the reported CART19 trials test the anti-CD19 scFv originated from FMC63, including the four CD19 CARs approved by the FDA. The anti-FMC63 antibody, which specifically identifies the scFv of anti-CD19 CARs derived from FMC63, has greater specificity and sensitivity.
Thus, the anti-FMC63 antibody is a better choice for the clinical samples with a lesser positive rate. Yet, the development of high-affinity CD19 protein and anti FMC63 antibody always faces a great challenge and there is only a handful of products that are currently commercially available.
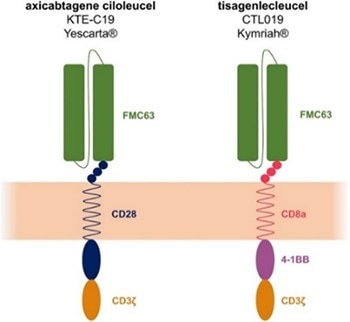
Image Credit: ACROBiosystems
ACROBiosystems devised a complete series of CD19 protein products, such as biotinylated CD19, unconjugated CD19 and fluorescent-labeled CD19. These proteins can efficiently identify the expression of anti-CD19 CAR on the surface of the transduced T cells and have been broadly employed for the quality control release testing and pharmacokinetic (PK) research of anti-CD19 CAR-T cells.
Simultaneously, ACRO has also created the high-qualified monoclonal anti-FMC63 scFv antibodies, the performance of which is well validated in-house by flow cytometry, indicating suitability for identification of FMC63 derived anti-CD19 CARs in clinical trials.
Results
CD19 protein
High specificity
PE-labeled CD19 can particularly identify CD19 CAR expression by FACS.
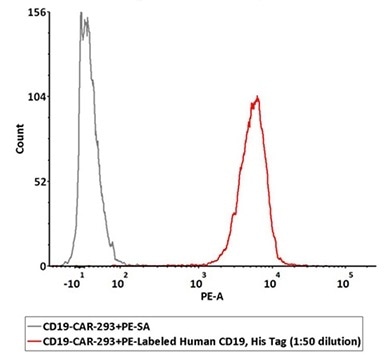
1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was used as a negative control. Image Credit: ACROBiosystems
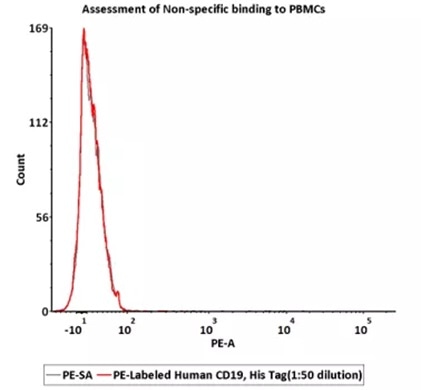
5e5 of PBMCs were stained with 100 μL of 1:50 dilution of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3), PE signal was used to evaluate the binding activity. Image Credit: ACROBiosystems
High bioactivity
Greater binding affinity compared to competitive products verified by ELISA and FACS.
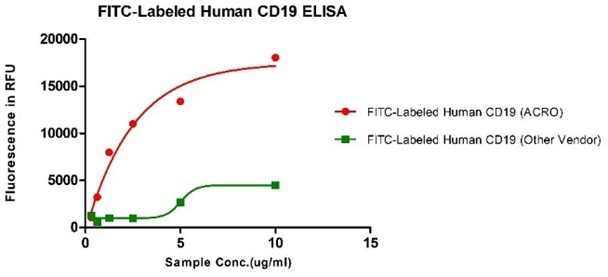
Human CD19, His Tag from two different vendors were evaluated in the ELISA analysis against FMC63 Mab. The result showed that ACRO’s FITC-Labeled Human CD19, His Tag has a much higher binding activity than that of the other vendor. Image Credit: ACROBiosystems
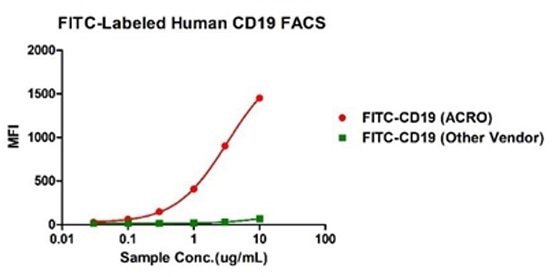
Binding activity of FITC-Labeled Human CD19, His Tag from two different vendors were evaluated in the flow cytometry analysis against anti-CD19-CAR-293 cells. The result showed that ACRO's FITC-Labeled Human CD19, His Tag has a much higher binding activity than that of the other vendor. Image Credit: ACROBiosystems
Anti-FMC63 scFv antibody
High sensitivity and specificity
No background staining when identifying FMC63 derived Anti-CD19 CARs in clinical trials.
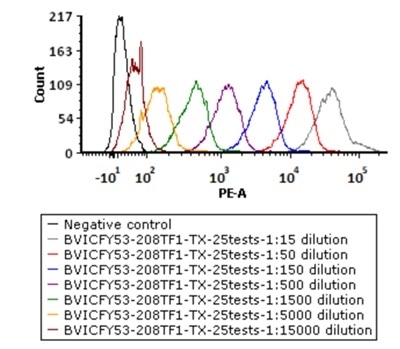
5e5 of Anti-CD19 CAR-293 cells were stained with a series of concentrations of PE-Labeled Monoclonal Anti FMC63 scFv Antibody, Mouse IgG1(Cat. No. FM3-HPY53), and negative control respectively. PE signal was used to evaluate the binding activity. Image Credit: ACROBiosystems
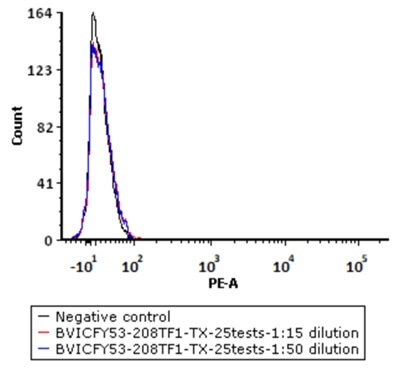
5e5 of Human PBMC cells were stained with a series of concentrations of PE-Labeled Monoclonal Anti FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-HPY53), and negative control respectively. PE signal was used to evaluate the binding activity. Image Credit: ACROBiosystems
High stability
Stable at 37 ℃ for 72 hours, 4 ℃ for 14 days and −70 ℃ for 6 years with high bioactivity.
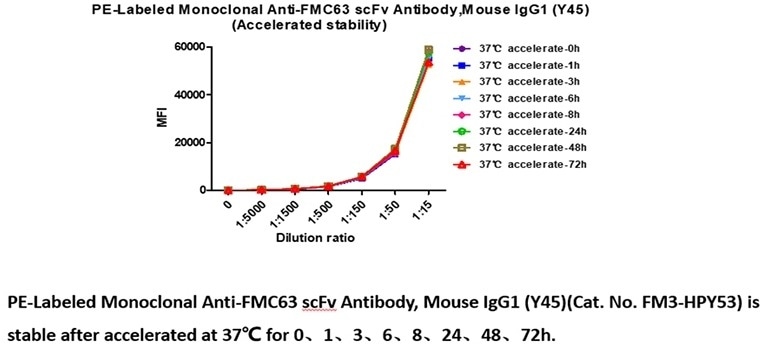
PE-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Y45) (Cat. No. FM3-HPY53) is stable in undiluted samples at 37 ℃ for 72 hours, equivalent to store at 4 ℃ for 14 days without performance reduction and equivalent to store at −70 ℃ for 83 months without performance reduction. Image Credit: ACROBiosystems
High batch-to-batch consistency
The binding activity of various kinds of anti-FMC63 antibody was detected by FACS.
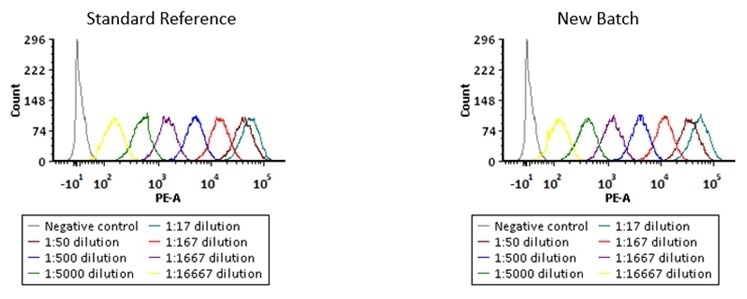
Binding activity of two different lots of PE-Labeled Monoclonal Anti FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-HPY53) against Anti-CD19 CAR-293 cells was evaluated by flow cytometry. The result shows very high batch-to-batch consistency. Image Credit: ACROBiosystems
Conclusion
CAR-T cells product is a type of a living drug, and it needs whole-process quality control, like inspection of the materials employed in production, process control and a release test of the completed products.
Analyzing CAR expression is a vital step in the quality control of CAR-T cells. ACRO’s CD19 proteins and anti FMC63 antibody products have great sensitivity and specificity, making them the perfect tools for quality control release testing and pharmacokinetic research.
Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.