The U.S. Food and Drug Administration (FDA) announced a landmark decision on April 10, 2025, phasing out mandatory animal testing for drugs such as monoclonal antibodies.
This revolutionary decision seeks to adopt New Approach Methodologies (NAMs) able to better and more efficiently reflect human biology.
The FDA aims to improve drug development safety and efficiency by reducing reliance on animal testing, effectively accelerating the approval of new therapies, and promoting a more ethical, human-relevant global research paradigm.

Image Credit: https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs
Key policy highlights
Objective
By gradually eliminating mandatory animal testing in the development of monoclonal antibodies and other drugs, the FDA aims to pivot toward more human-relevant alternatives such as organoids, organ-on-a-chip technologies, and AI-based toxicity prediction models.
Scope
These changes come into effect immediately for new drug IND submissions, with sponsors now encouraged to submit NAM data to support safety assessments.
Global data acceptance
Human safety data will be accepted if it comes from countries with comparable regulatory standards, reducing redundant animal testing.
Regulatory incentives
Streamlined FDA review processes may be available to companies providing robust, scientifically validated NAM data.
Upcoming pilot program
The FDA will launch a pilot program within the next year. This program will focus on monoclonal antibody development driven by non-animal testing, aiming to gather appropriate experience and data to inform future regulatory updates.
Organoids enter a golden era
The recently released policy highlights regulators' increasing emphasis on human-relevant data. It also sends a clear signal to the industry, stressing that organoid-based human models are quickly evolving from basic research into being central to preclinical drug evaluation.
Organoids are lab-grown, three-dimensional mini-organs derived from human cells. Organoids can closely mimic the structure and function of real human organs, offering demonstrable advantages in drug screening, toxicity testing, and disease modeling.
Organoids offer greater physiological relevance and predictive accuracy than traditional animal models, positioning them as a vital alternative technology with the potential to underpin the future of drug development.
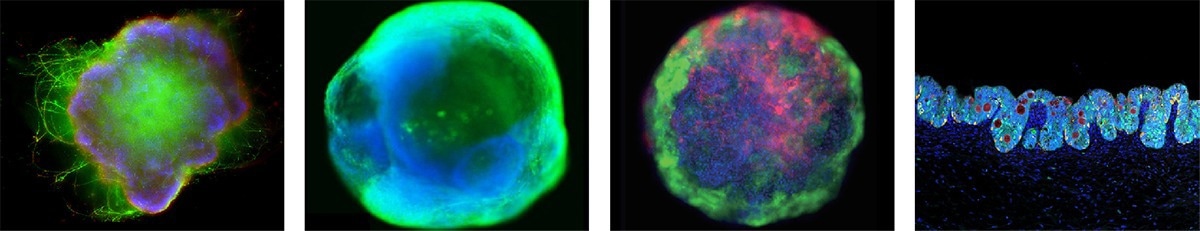
ACROBiosystems' brain, cardiac, liver, and intestinal organoids. Image Credit: ACROBiosystems
ACROBiosystems’ comprehensive organoid toolbox
ACROBiosystems is an industry leader in the field of organoids, with its Organoid Toolbox representing the most relevant solution in this emerging area.
This comprehensive solution features a range of ready-to-use and cryopreserved iPSC-derived organoids and organoid differentiation kits. Customized organoid development, disease modeling, and analytical services are also included, empowering drug discovery and disease research throughout the field.
The company’s featured products include:
- Cardiac organoids (Cat. No. CIPO-HWL002K) suitable for the assessment of cardiac toxicity and early safety evaluation
- Cerebral organoids (Cat. No. CIPO-BWL001K), incubated with pre-formed fibrils (PFFs), suitable for modeling neurodegenerative diseases due to their capacity to effectively recapitulate key pathological features of conditions such as Parkinson's and Alzheimer's disease.
- Liver organoids (Cat. No. CIPO-RWL005K) suitable for establishing non-alcoholic steatohepatitis (NASH) models and performing drug metabolism studies
- Intestinal organoids (Cat. No. CIPO-IWL003K) designed to offer an ideal in vitro model for the study of intestinal immune responses and inflammatory diseases
Cardiac Organoids. Image Credit: ACROBiosystems
Brain Organoids. Image Credit: ACROBiosystems
Liver Organoids. Image Credit: ACROBiosystems
Intestinal Organoids. Image Credit: ACROBiosystems
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.