The Notch signaling pathway is a highly maintained intercellular signaling pathway that involves multiple processes of growth and development, like apoptosis, cell proliferation, differentiation of pluripotent progenitor, and formation of cell boundaries.
Delta‐like canonical Notch ligand 3 (DLL3) belongs to the Notch receptor ligand family and is involved in Notch signaling. DLL3 has been found to be strongly expressed in small cell lung cancer (SCLC) and other neuroendocrine tumors, but it is rarely expressed in normal tissues.
Hence, it has gained huge interest from people as a new target for cancer treatment. Several researchers are trying to find ways to target DLL-3 for the precision treatment of lung cancer.1
Structure and function of DLL3
Human DLL3 is a single transmembrane protein with 619 amino acids that adhere to the cell surface. A DSL domain, an intracellular domain, and six epidermal growth factor-like domains make up the entire structure.
DLL3 is commonly expressed in the Golgi apparatus and shows up on the cell surface when overexpressed, unlike other Notch ligands. DLL3 does not attach to the Notch receptor and is instead considered a cell-autonomous Notch signaling inhibitor.2,3
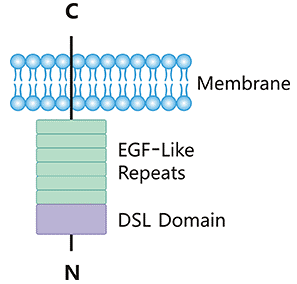
Figure 1. DLL3 structure. Image Credit: ACROBiosystems
DLL3 and cancer
DLL3 and lung cancer
DLL3 is greatly expressed on the surface of small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC), according to recent research. As of now, ROVA-T, an ADC drug that targets DLL3, is a hopeful targeted therapy drug that has shown efficacy in the treatment of SCLC and LCLNEC.
DLL3 inhibition prevents the development of the two types of cells mentioned above and causes apoptosis.
DLL3 was also discovered to be a direct downstream target of ASCL1, a transcription factor linked to the development of pulmonary neuroendocrine cells, implying that DLL3 is linked to neuroendocrine tumorigenesis, particularly in lung cancer-related neuroendocrine tumors.4,5,6
But the development of ROVA-T was suspended as a result of the short overall survival (OS) in comparison to the Topotecan as the positive control.7,8,9 Clinical data suggests that DLL3 is overexpressed in over 80% of small cell lung cancer patients. The high expression of DLL3 in SCLC has been correlated negatively with the survival time of patients.
DLL3 and liver cancer
DLL3 gene expression can be silenced in hepatocellular carcinoma (HCC) cells due to epigenetic modifications like abnormal DNA methylation and histone acetylation. Histone deacetylase inhibitors enable DLL to be expressed in HCC cells, which inhibits cell growth and causes apoptosis.
In hepatitis B virus (HBV)-associated HCC, the hepatitis B virus protein HBx could also induce epigenetic modifications and impede DLL3 expression. DLL3 expression might very well show different trends in various malignancies, despite the fact that it is a therapeutic target because of its expression in some cancers.11,12
Novel targeted DLL3 therapy
Bispecific antibodies, cell therapy, antibody-coupled drugs (ADCs), monoclonal antibodies, and bispecific T-cell conjugates (BiTE) are just a few of the therapeutic approaches targeting DLL3 that have recently been developed and advanced to clinical trials with proven therapeutic effects.
Near-infrared immunotherapy
This method employs antibody photosensitizer conjugates that irradiate and damage cancer cells using near-infrared light. The ROVA-T antibody-coupled IR700 photosensitizer is substantially dissolved under near-infrared light irradiation in cells incubated with ROVA-IR700. Furthermore, ROVA-IR700M-treated mice exhibited significant xenograft shrinkage.13
Bispecific T-cell conjugates
Bispecific T‐cell engager (BiTE) is a new immunotherapy technique that redirects a patient’s T-cells to kill tumor cells. The AMG 757, developed by Amgen, has been improved to expand the biTE molecular half-life.
This triggers T cells to target tumors expressing DLL3. AMG 757 has been confirmed to be effective against SCLC cell lines in vitro; in mouse models.
AMG 757 considerably resolves patient-derived xenografts and in situ SCLC tumors by triggering T cells. It indicates that AMG 757 has been anticipated to be a novel SCLC immunotherapy drug targeting DLL3. The AMG 757 Clinical Phase I study (NCT03319940) is ongoing for SCLC patients.14
CAR-T cell therapy
Antigen receptor chimeric T cell therapy entails genetically transforming a patient’s own T cells to direct them to express tumor antigen-specific chimeric receptors. The cells are then reinjected into the patient to attack and kill the target cells.
AMG 119 is an adoptive cell therapy in which a patient’s autologous T cells are modified to express a DLL3 transmembrane chimeric receptor (CAR) that attacks DLL3-positive cells.
In vitro assays showed that AMG 119 CAR-T cell therapy can inhibit SCLC xenograft tumor growth, and in vivo validation showed that AMG 119 CAR-T cell therapy can decrease SCLC cells expressing DLL3. A Phase I clinical trial (NCT03392064) is currently underway to assess the safety, tolerability, and efficacy of AMG 119 in the treatment of SCLC.15
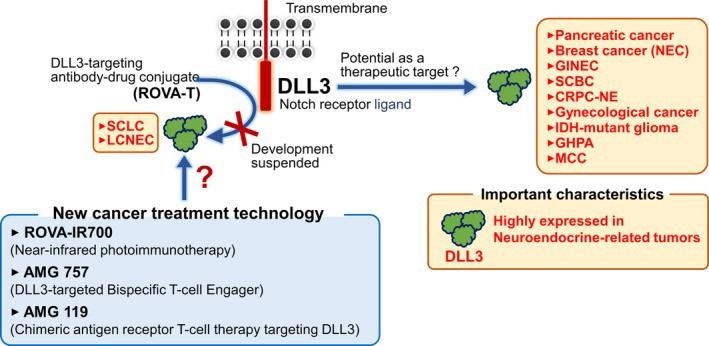
Figure 2. Expression and therapeutic potential of DLL3 in malignant tumors. Image Credit: ACROBiosystems
To facilitate drug development targeting DLL3, ACROBiosystems provides a range of high-quality DLL3 protein products.
Features
- More species: Human, Rat, Mouse, Rhesus macaque and Cynomolgus can all be fully applied for the cross-examination of various species
- Higher purity: SDS-PAGE verified purity >95%, SEC-MALS verified purity >90%
- More tags: Provide His, His and Avi, Fc, Fc and Avi tag products to fulfill different experimental requirements.
Product list. Source: ACROBiosystems
| Molecule |
Cat. No. |
Species |
Product Description |
| DLL3 |
DL3-H52H4 |
Human |
Human DLL3 Protein, His Tag (MALS verified) |
| DLL3 |
DL3-H5255 |
Human |
Human DLL3 Protein, Fc Tag (MALS verified) |
| DLL3 |
DL3-HF2H4 |
Human |
FITC-Labeled Human DLL3 Protein, His Tag |
| DLL3 |
DL3-HF253 |
Human |
FITC-Labeled Human DLL3 Protein, Fc Tag |
| DLL3 |
DL3-M53H9 |
Mouse |
Mouse DLL3 Protein, His Tag |
| DLL3 |
DL3-C52H3 |
Cynomolgus |
Cynomolgus DLL3 Protein, His Tag |
| DLL3 |
DL3-R52H3 |
Rat |
Rat DLL3 Protein, His Tag |
| DLL1 |
DL1-H52H8 |
Human |
Human DLL1 / Delta1 Protein, His Tag |
| DLL4 |
DL4-H5259 |
Human |
Human DLL4 Protein, Fc Tag |
| DLL3 |
DL3-H82E4 |
Human |
Biotinylated Human DLL3 Protein, His, Avitag™ (MALS verified) |
| DLL3 |
DL3-H82F5 |
Human |
Biotinylated Human DLL3 Protein, Fc, Avitag™ |
| DLL3 |
DL3-R52H4 |
Rhesus macaque |
Rhesus macaque DLL3 Protein, His Tag |
| NOTCH1 |
NO1-M52H8 |
Mouse |
Mouse NOTCH1 Protein, His Tag |
| NOTCH2 |
NO2-H5255 |
Human |
Human NOTCH2 Protein, Fc Tag (MALS verified) |
| NOTCH3 |
NO3-H5255 |
Human |
Human NOTCH3 Protein, Fc Tag |
Assay data
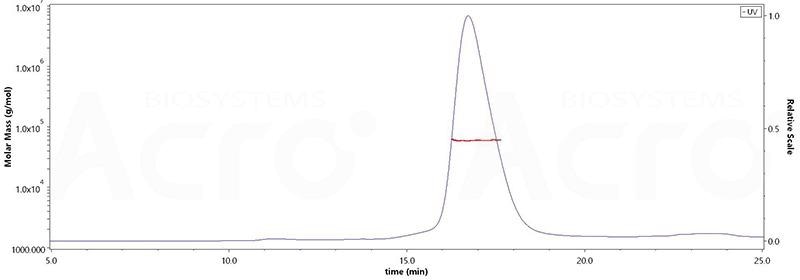
The purity of Human DLL3, His Tag (Cat.No. DL3-H52H4) is over 95% and the molecular weight of this protein is around 55–75 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
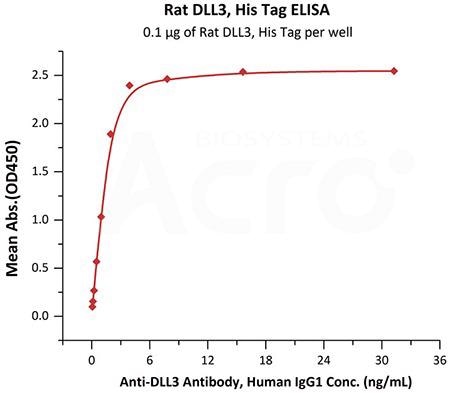
Immobilized Rat DLL3, His Tag (Cat. No. DL3-R52H3) at 1 μg/mL (100 μL/well) can bind Anti-DLL3 Antibody, Human IgG1 with a linear range of 0.1-4 ng/mL (QC tested). Image Credit: ACROBiosystems
References
- Matsuo K, Taniguchi K, Hamamoto H, Inomata Y, Komura K, Tanaka T, et al. Delta-like canonical Notch ligand 3 as a potential therapeutic target in malignancies: A brief overview. Cancer Science. 2021;112(8):2984-92.
- Steinbuck, Martin Peter, and Susan Winandy. "A review of notch processing with new insights into ligand-independent notch signaling in T-cells." Frontiers in immunology 9 (2018): 1230.
- Hu, Bingxin, et al. "Over-expression of human Notch ligand Delta-like 3 promotes proliferation of human gastric cancer cells in vitro." Nan Fang yi ke da xue xue bao= Journal of Southern Medical University 38.1 (2018): 14-19.
- Augustyn A, Borromeo M, Wang T, Fujimoto J, Shao C, Dospoy PD, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proceedings of the National Academy of Sci U S A. 2014;111(41):14788-93.
- Geffers I, Serth K, Chapman G, Jaekel R, Schuster-Gossler K, Cordes R, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. Journal of cell Biology Cell Biology. 2007;178(3):465-76.
- Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Human Molecular Genetics. 2011;20(5):905-16.
- Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncology. 2017;18(1):42-51.
- Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Science Translational Medicine. 2015;7(302):302ra136.
- Matsuo K, Taniguchi K, Hamamoto H, Ito Y, Futaki S, Inomata Y, et al. Delta-like 3 localizes to neuroendocrine cells and plays a pivotal role in gastrointestinal neuroendocrine malignancy. Cancer Science 2019;110(10):3122-31.
- Bauer, Todd M., et al. "ORAL02. 01: safety and efficacy of single-agent rovalpituzumab tesirine, a DLL3-targeted ADC, in recurrent or refractory SCLC: topic: medical oncology." Journal of Thoracic Oncology 11.11 (2016): S252-S253.
- Mizuno Y, Maemura K, Tanaka Y, Hirata A, Futaki S, Hamamoto H, et al. Expression of delta-like 3 is downregulated by aberrant DNA methylation and histone modification in hepatocellular carcinoma. Oncology Reports. 2018;39(5):2209-16.
- Hamamoto H, Maemura K, Matsuo K, Taniguchi K, Tanaka Y, Futaki S, et al. Delta-like 3 is silenced by HBx via histone acetylation in HBV-associated HCCs. Scientific Reports. 2018;8(1):4842.
- Isobe Y, Sato K, Nishinaga Y, Takahashi K, Taki S, Yasui H, et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine. 2020;52:102632.
- Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clinical Cancer Research. 2021;27(5):1526-37.
- Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. "Journal of Hematology & Oncology. 2019;12(1):61
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.