ACROBiosystems established an emergency response team to help address the lab and industrial need for viral mutant proteins in response to the prevalence of the Omicron variant of COVID-19.
Over the course of 10 days, the company was able to prepare and stock a portfolio of high-quality Omicron antibodies, antigens and other reagents at the gram level.
Timeline of Omicron development
This rapidly developed product portfolio was in direct response to the rapid onset of the Omicron variant.
- November 24th: The B.1.1.529 variant was discovered for the first time in South Africa. This was reported to the WHO after being found related to the soaring cases in the country that month.
- November 26th: The WHO hosted an emergency meeting to look at the impact of B.1.1.529. This was designated Omicron and identified as the fifth variant of concern (VOC).
- December 8th: The WHO confirmed that the variant had been found in 57countries.
- December 13th: In the UK, the first death of a patient with Omicron was reported.
Diagnosis of Omicron
The Food and Drug Administration (FDA) revised the EUAs of a number of molecular, antigen and serology tests to establish extra Conditions of Authorization. This was on September 23rd, 2021, in response to the continued emergence of new COVID-19 variants.
This revision prompts and requires the developers of tests for COVID-19 to update their authorized labeling and assess the impact of SARS-CoV-2 viral mutations on test performance.
Should mutations be identified as affecting the test performance, the EUA holder is required to communicate this to the FDA and end-users. Due to the sheer number of mutations present in the Omicron variant, its impact on current diagnostic reagents is of great concern.
Molecular tests
The deletion of HV69-70 amino acids within Omicron’s spike gene can lead to an undetectable S-gene target or S-gene target failure (SGTF) when assessed via specific real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing methods.
This factor makes these tests highly predictive of Omicron.
The emergence of the Omicron variant has led to a rapid and significant increase in the proportion of SARS-CoV-2 samples where SGTF has been identified. This has enabled an estimate of Omicron’s growth rate.
Recent reports have discovered a number of Omicron sub-lineages that have evolved to miss the SGTF marker, potentially compromising convention PCR methods’ detection efficiency.
Antigen tests
Antigen tests represent a crucial complementary method to molecular tests when screening for SARS-CoV-2 variants.
It is anticipated that antigen detection methods able to efficiently detect Omicron mutant strains will play a vital role in the detection, prevention and control of Omicron.
Antibody tests
As immunization coverage increases via natural infection or vaccination, the significance of serological antibody tests has become more and more prominent.
Tests able to detect antibodies to the SARS-CoV-2 virus – for example, IgM and IgG – offer a direct and reliable means of assessing the degree of herd immunity present in a population.
ACROBiosystems is the world’s leading supplier of protein reagents for COVID-19 detection.
The company has been committed to supporting the development of highly sensitive and specific diagnostic tools since the initial outbreak of COVID-19, with its high-quality products and excellent service recognized by many of its IVD partners.
ACROBiosystems can provide a number of products designed for the development of Omicron-specific diagnostic reagents, including:
- Spike trimer/RBD/S1/N proteins featuring guaranteed bioactivity and high
- High-sensitivity nucleocapsid antibody pairs able to detect Omicron-N at 0.39 ng/mL
- Highly sensitive N antibody pairs designed to ensure efficient detection of Omicron antigens
All of these products are available in gram-level supplies.
Verification using a colloidal gold platform confirms that ACROBiosystems’ SARS-CoV-2 nucleocapsid antibody pair (Cat. No. NUN-S95/NUN-M223) is able to detect the Omicron mutant strain with high sensitivity and accuracy.
The detection limit of Omicron is consistent with detection limits of the wild type (0.39 ng/mL), ensuring there is no impact on the detection efficiency of antigen test products developed with pair of antibodies at their core.

Image Credit: ACROBiosystems
Historical data has also provided robust validation of the advanced performance of SARS-CoV-2 nucleocapsid antibody pair (Cat. No. NUN-S95/NUN-M223) in both sandwich ELISA and lateral flow assays (LFA).
The company’s products are able to detect all VOC and VOI mutants at 0.195-2 ng/mL sensitivity in colloidal gold-based immunoassays, while its sensitivity when detecting antigen content in inactivated vaccine samples is as low as a 1:38400 dilution ratio.
Most notably, this tool is specific for SARS-CoV-2 and exhibits no cross-reactivity with any of the other five human-infecting coronaviruses.
Its verifiable high specificity and sensitivity illustrate the suitability of this antibody pair in establishing a rapid antigen detection method – an essential factor in preventing and controlling the spread of the Omicron variant.
Summary
Highly quality antigens are key to the development of Omicron antibody detection tools, and ACROBiosystems has developed all the necessary antigen proteins required, including full-length spike trimer, S RBD, NTD, S1 and nucleocapsid proteins.
The company is committed to developing high-quality technologies and products to address the urgent requirements of the IVD industry.
Consistent product quality, approachable, knowledgeable technical services and a stable global supply for all customers and partners help make ACROBiosystems the global leader in its field.
Omicron spike RBD protein (Cat.No. SPD-C522e)
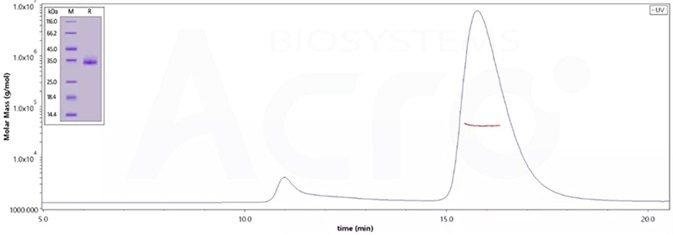
The purity of (Cat.No. SPN-C52Hz) is 95% as determined by SDS-PAGE and 96.8% as determined by SEC-MALS. Image Credit: ACROBiosystems
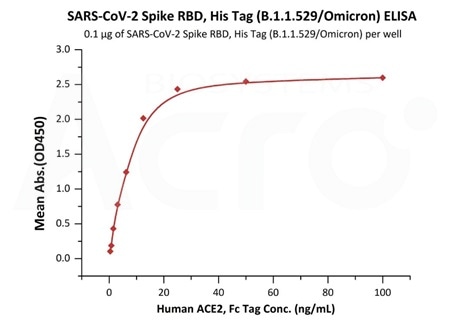
Measured by its binding ability in a functional ELISA. Immobilized Human ACE2, Fc Tag (Cat.No. AC2-H5257) at 5 μg/mL (100 μL/well) can bind SARS-CoV-2 Spike Trimer, His Tag (B.1.1.529/Omicron) (Cat.No. SPN-C52Hz) with a linear range of 0.08-1 ng/mL. Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.