Fc engineering has many important uses in antibody drug development. It enhances antibody-dependent cellular cytotoxicity (ADCC) in the tumor microenvironment to increase tumor cell-killing efficacy and suppresses complement activation that may occur during autoimmune disease treatment. This reduces adverse drug reactions, extends antibody serum half-life by optimizing FcRn binding properties to reduce dosing frequency, and allows tissue-specific antibody delivery to improve drug accumulation in target tissues.
According to authoritative data from Nature Reviews Drug Discovery (2023), 87 % of the 1,200 antibody therapies now in global research include Fc engineering, a proportion that has increased by 3.2 times since 2018. This highlights Fc engineering's critical significance and expanding trajectory in antibody medication research and development.
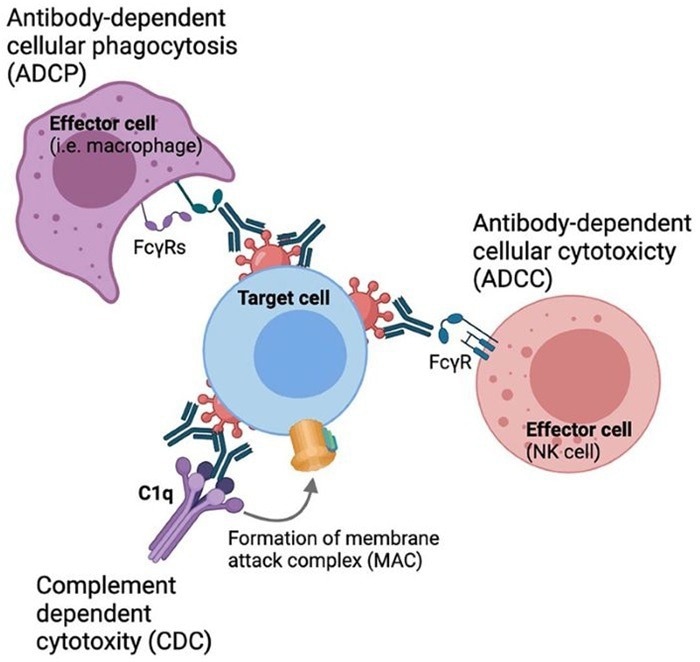
Fc-mediated effector functions. Image Credit: ACROBiosystems
Fc engineering strategy analysis and quality control with isotype controls
IgG1: Functional versatility and balancing strategies
IgG1 is the "workhorse" of therapeutic antibody development, accounting for 65 % of commercialized antibody therapies. Its Fc domain has 12 key functional regions that can be precisely modulated using "molecular switch" alterations.
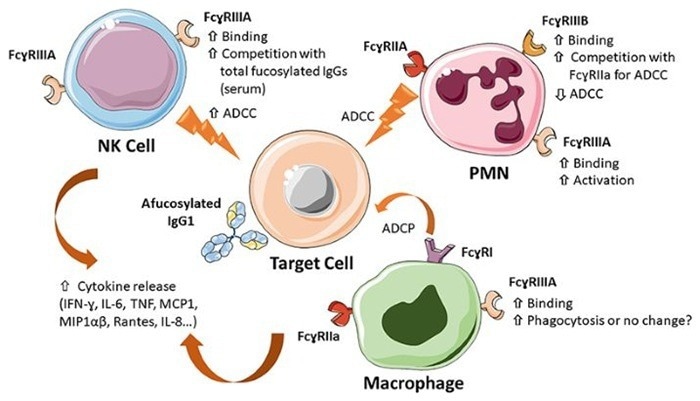
Major mechanisms of action of Fc core afucosylated IgG1 antibodies. Image Credit: ACROBiosystems
Numerous successful Fc engineering case studies in clinical settings have robustly proven the strategy's efficacy. With the D239E/L241E double mutation, Roche's Ocrelizumab has a 40-fold higher Fc binding affinity to FcγRIIIa.
This dramatically increased the antibody's B-cell depletion efficiency, resulting in a more effective therapeutic approach for relevant disorders. Durvalumab, developed by AstraZeneca, uses the L234F/L235E/P331S triple mutation, which reduces complement activation capacity to 0.01 % of wild-type levels.
This successfully mitigates the risks associated with complement activation. Sanofi's Nirsevimab, which includes the M252Y/S254T/T256E mutations, has a 10-fold increase in FcRn binding affinity at pH 6.0.
This increased the serum half-life to 72 days, significantly reducing patient dose frequency and boosting treatment adherence.
Choosing adequate isotype controls is crucial for appropriately analyzing the functional properties of modified antibodies. Products such as recombinant IgG1 Fc protein and IgG1 antibody isotype controls serve as accurate reference benchmarks for research, assuring precision and reproducibility.
IgG2: Natural effector functions and engineering approaches
Although IgG2 has relatively limited natural effector activities, additional engineering may be necessary to completely inactivate residual activity in specific applications. For example, Remanezumab, a migraine-targeting anti-CGRP antibody, contains A330S/P331S double mutations that reduce complement binding capacity, ensuring that the medicine only functions through signal-blocking pathways.
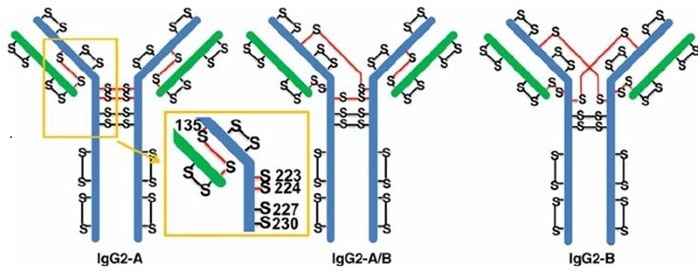
IgG2 and its disulfide isoforms. Image Credit: ACROBiosystems
Using matched isotype controls, such as recombinant IgG2 Fc protein and IgG2 antibody isotype controls, prevents structural mismatch interference. This assures experimental validity and precise data support for drug development.
IgG4: Hinge stability engineering and low effector function profiles
The Fab arm of IgG4 exchange capability previously caused safety concerns, but engineering improvements have resulted in a stunning metamorphosis.
Merck's Pembrolizumab uses an S228P mutation to improve stability and safety by reducing aggregation rates after 28 days of storage at 37 °C and decreasing FcγRIIa binding activity.
Sanofi's Dupilumab fine-tunes effector functions using S228P/L235E double mutations to reduce residual ADCC activity to insignificant levels, lowering the risk of adverse reactions.
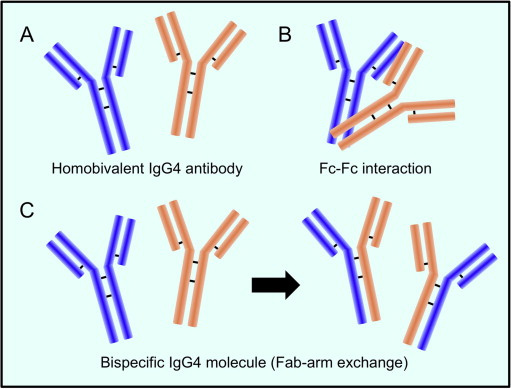
Structural uniqueness of IgG4 antibodies. Image Credit: ACROBiosystems
When developing IgG4 antibodies, selecting proper isotype controls is crucial for correct evaluation. ACROBiosystems’ recombinant IgG4 Fc protein and IgG4 antibody isotype controls fulfill this need, providing dependable reference standards for drug research.
IgG3 and IgE: Engineering specialized subtypes
IgG3 has a prolonged hinge region (62 amino acids, four times longer than IgG1), which allows for robust complement activation but also makes it prone to metalloprotease cleavage. The serum half-life of seven days restricts its clinical relevance.
IgE's Fc domain binds to FcεRI receptors with high affinity, allowing for precise targeting. Novartis' Omalizumab targets the Cε3 domain of free IgE, lowering mast cell surface IgE receptor occupancy by 95 %, providing an effective treatment for allergic disorders.
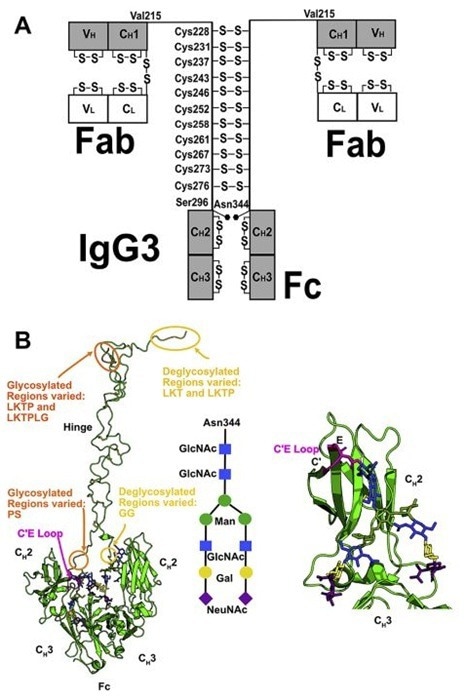
Human IgG3 domains and glycosylation. Image Credit: ACROBiosystems
ACROBiosystems’ recombinant IgG3 Fc protein, IgE Fc protein, and IgG3 antibody isotype controls serve as crucial benchmarks for engineering these specialized subtypes.
These solutions provide solid reference data for drug development, allowing for better insights into the properties and activities of specialized antibodies while also expediting therapeutic innovation.
ACROBiosystems' comprehensive Fc isotype control solutions for biopharmaceutical innovation
Fc engineering is crucial throughout antibody drug development, allowing for critical functional optimizations such as increased tumor-killing capacity and cytokine storm prevention, as well as serum half-life extension and structural stability improvements.
ACROBiosystems provides dual-format isotype control products that include wild-type and mutant Fc variants. The native wild-type Fc protein maintains its original shape, making it appropriate for basic research and control tests.
The mutant Fc is generated using clinically confirmed antibody mutation sites and is precisely tuned to suit the engineering requirements of more than 20 FDA-approved antibody therapies.
His/Flag/Biotin/Avitag™ tags are versatile and compatible with multiple applications, including FACS, ELISA, and SPR. ACROBiosystems’ solutions offer comprehensive, customized support for antibody drug R&D and validation, accelerating discoveries and advances in the biopharmaceutical industry.

Image Credit: ACROBiosystems

Image Credit: ACROBiosystems

Image Credit: ACROBiosystems

Image Credit: ACROBiosystems
Reference
- Phelps, M. and Balazs, A.B. (2021). Contribution to HIV Prevention and Treatment by Antibody-Mediated Effector Function and Advances in Broadly Neutralizing Antibody Delivery by Vectored Immunoprophylaxis. Frontiers in Immunology, 12. https://doi.org/10.3389/fimmu.2021.734304.
- Golay, J., Andrea, A.E. and Cattaneo, I. (2022). Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.929895.
- Jones, L.M., et al. (2013). Complementary MS Methods Assist Conformational Characterization of Antibodies with Altered S–S Bonding Networks. Journal of the American Society for Mass Spectrometry, 24(6), pp.835–845. https://doi.org/10.1007/s13361-013-0582-4.
- Moriyama, M., et al. (2014). T helper subsets in Sjögren’s syndrome and IgG4-related dacryoadenitis and sialoadenitis: A critical review. Journal of Autoimmunity, 51, pp.81–88. https://doi.org/10.1016/j.jaut.2013.07.007.
- Spiteri, V.A., et al. (2021). Solution structures of human myeloma IgG3 antibody reveal extended Fab and Fc regions relative to the other IgG subclasses. Journal of Biological Chemistry, 297(3), pp.100995–100995. https://doi.org/10.1016/j.jbc.2021.100995.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.