Claudin18.2 is a subtype of the Claudin18 tight junction protein family, found on the surface of cell membranes.
Under normal conditions, its expression is limited to low levels in differentiated epithelial cells of the gastric mucosa. However, in pathological states, Claudin18.2 becomes significantly overexpressed across a range of tumors—including gastric, pancreatic, gallbladder, biliary tract, esophageal, and lung cancers—positioning it as a promising therapeutic target for a wide patient population.
Several treatment approaches are currently in development to target Claudin18.2, including monoclonal antibodies, bispecific antibodies, antibody-drug conjugates (ADCs), and chimeric antigen receptor T-cell (CAR-T) therapies. Many of these candidates are already progressing through different phases of clinical research.
In clinical trials, Claudin18.2 expression is a critical factor for determining patient eligibility. Data consistently show that patients with high Claudin18.2 positivity in immunohistochemistry (IHC) testing are more likely to respond favorably to targeted treatments.
As a result, accurate screening of Claudin18.2 expression via IHC not only improves the efficiency and success rate of clinical trials but also plays a key role in guiding treatment selection and drug development strategies.
However, the presence of another subtype of Claudin18, Claudin18.1, poses a significant challenge to achieving specificity in IHC detection of Claudin18.2.
Claudin18.1 and Claudin18.2 have highly similar structures, differing by only seven amino acid residues in the extracellular loop 1 (ECL1) sequence. Yet, their expression statuses in tumors are markedly different, with Claudin18.1 highly expressed only in normal lung alveolar tissue. The distinction between these two subtypes is crucial in cancer diagnosis and treatment, as they may have different clinical implications.
The study discussed in this article aimed to develop a specific IHC antibody that recognizes Claudin18.2 without cross-reacting with Claudin18.1. The antibody would be used to accurately assess Claudin18.2 expression in tumor tissues, offering strong support for determining patient eligibility and guiding clinical diagnosis.
Methods and materials
The study used human Claudin18.2 protein with a specific sequence to immunize mice, generating antigen-specific B cells. Hybridoma antibodies were then produced by fusing these B cells with myeloma cells, and the resulting antibodies were purified from ascites fluid using protein A affinity chromatography.
For antibody preparation and testing, a range of tissues was used, including normal gastric and lung tissue, gastric cancer, pancreatic cancer, colorectal cancer, as well as normal and abnormal human tissue microarrays.
Initial validation of the Claudin18.2 antibody (ACRO 3B10) was conducted via immunohistochemistry (IHC), focusing on cross-reactivity, specificity (immunoreactivity), accuracy, and various tumor indications.
Immunohistochemistry experimental method
Formalin-fixed, paraffin-embedded tissue samples were used for IHC detection of Claudin18.2. These samples were co-incubated with either the Claudin18.2 monoclonal antibody or a negative control reagent (NCR).
This was followed by sequential incubation with a specific secondary antibody and a ready-to-use chromogenic reagent containing secondary antibody molecules conjugated to a dextran polymer backbone and horseradish peroxidase (HRP). The addition of a chromogen substrate triggered enzyme catalysis, resulting in visible deposits at antigen sites.
A coloring enhancer was used to intensify the chromogenic signal. Finally, the samples were counterstained, coverslipped, and examined under an optical microscope.
Immunohistochemical scoring criteria
Target cells exhibit membrane staining. Normal gastric mucosal tissue is used as both a positive and negative control: differentiated gastric mucosal epithelial cells display strong membrane staining, while undifferentiated gastric stem cells, stromal lymphocytes, smooth muscle cells, and similar cell types show no staining.
Among the differentiated cells, chief cells demonstrate the strongest staining, the surface pit epithelium shows slightly lower intensity, and parietal cells exhibit the weakest staining—though still clearly positive, even at low intensity. Non-specific or background staining must be less than 1+.
Tumor cells are considered Claudin18.2-positive if any level of membrane staining is observed. Cytoplasmic staining alone is not regarded as positive. If fewer than 1 % of tumor cells show membrane staining, the result is classified as negative.
If 1 % or more of tumor cells are positive, the sample is considered positive overall. Furthermore, if 40 % or more of the tumor cells are graded as 2+ or 3+ in intensity, the sample is categorized as strong positive; if not, it is classified as weak positive.
Results
Assessment of cross-reactivity of the tissue sample against the Claudin-18.1 protein
Claudin18.1 and Claudin18.2 share a high degree of structural similarity, differing by only seven amino acid residues in the extracellular loop 1 (ECL1) sequence. Accurately distinguishing between these two subtypes is essential for effective cancer diagnosis and treatment.
Claudin18.2 is positively expressed in the gastric gland cells of normal gastric tissue but is absent in normal lung tissue. In contrast, Claudin18.1 is expressed in normal lung tissue and not in normal gastric tissue.
To evaluate potential cross-reactivity, a cross-reactivity assessment was carried out using one sample of normal gastric tissue and ten samples of normal lung tissue, based on the known expression pattern of Claudin18.2.
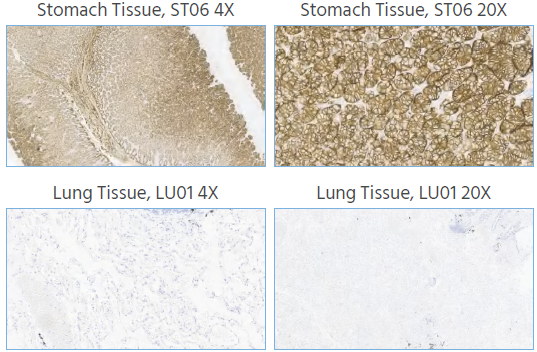
Figure 1. Representative IHC images show that Claudin 18.2 expression was detected in the stomach mucosa epithelial cells and absent from the lung tissue.IHC staining of human stomach and lung tissue was performed using Anti-Claudin18.2 at a dilution of 1:1000, with no background staining. Magnification, x4 and x20. Image Credit: Source: ACROBiosystems
Claudin18.2 staining in normal gastric tissue showed strong positive signals in the cytoplasm and/or cell membrane of gastric gland cells, while normal lung tissue showed no staining of lung cells.
This confirms that the Claudin18.2 antibodies do not cross-react with the Claudin18.1 protein. The staining results clearly differentiate between Claudin18.2 and Claudin18.1, minimizing the risk of cross-reactivity and improving the accuracy of Claudin18.2 detection.
Antibody specificity for multiple tissues
The ability to specifically recognize Claudin18.2—without interference from matrix proteins in different tissue types—is essential for reliable detection across various tumor indications. In this study, Claudin18.2-specific antibodies were used to perform an immunohistochemical specificity assessment using normal human tissue microarrays and tumor tissue microarrays.
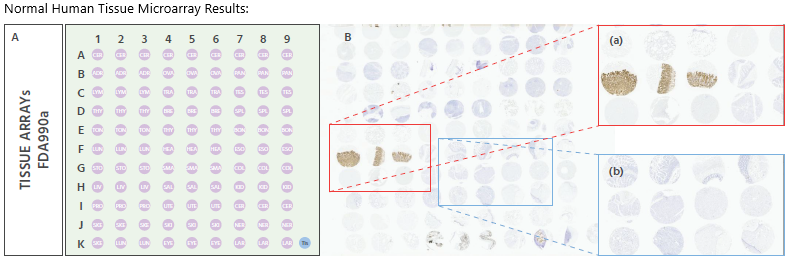
Figure 2. IHC staining of a normal human TMA (Pantomics #FDA990a), using Anti-Claudin18.2 at a dilution of 1:1000. It was detected in normal stomach tissue and non-staining of other tissues. The TMA includes 32 types of normal human organs: Cerebrum(6 cases), Cerebellum(3 cases), Adrenal gland(3 cases), Ovary(3 cases), Pancreas(3 cases), Lymph node(3 cases), Trachea(3 cases), Testis(3 cases), Thyroid gland(3 cases), Breast(3 cases), Spleen(3 cases), Tonsil(3 cases), Thymus gland(3 cases), Bone marrow(3 cases), Lung(3 cases), Heart(3 cases), Esophagus(3 cases), Stomach(3 cases), Small intestine(3 cases), Colon(3 cases), Liver(3 cases), Salivary gland(3 cases), Kidney(3 cases), Prostate(3 cases), Uterus(3 cases), Cervix(3 cases), Skeletal muscle(3 cases), Skin(3 cases), Nerve(3 cases), Skeletal muscle (1 cases), Lung(2 cases), Eye(3 cases), Larynx(3 cases). Magnification, x2. Image Credit: Source: ACROBiosystems
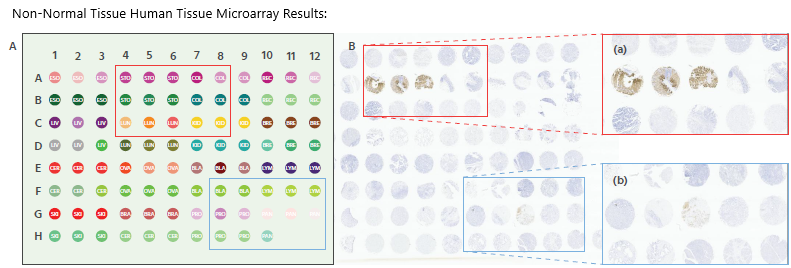
Figure 3. IHC staining of a non-normal human TMA (Pantomics # X096Mc01), using Anti-Claudin18.2 at a dilution of 1:1000. It was detected in normal stomach tissue and non-staining of other tissues. The TMA includes 16 types of normal human organs: Esophagus(6 cases), Stomach(6 cases), Colon(6 cases), Rectum(6 cases), Liver(6 cases), Lung(6 cases), Kidney(6 cases), Breast(6 cases), Cervix(6 cases), Ovary(6 cases), Bladder(6 cases), Lymph node(6 cases), Skin(6 cases), Brain(3 cases), Cerebrum(3 cases), Prostate(6 cases), Pancreas(6 cases). Magnification, x2. Image Credit: Source: ACROBiosystems
In normal gastric tissue, Claudin18.2 staining appeared as expected, with no non-specific expression observed.
This indicates that the antibody's staining is accurately localized, confirming its ability to specifically recognize Claudin18.2 across different tissue types. As a result, it effectively labels the target protein while minimizing interference from non-target proteins, thereby enhancing the overall accuracy of Claudin18.2 detection.
Claudin-18.2 expression intensity in cancer
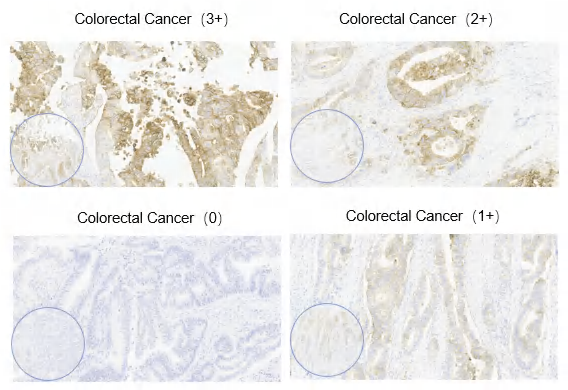
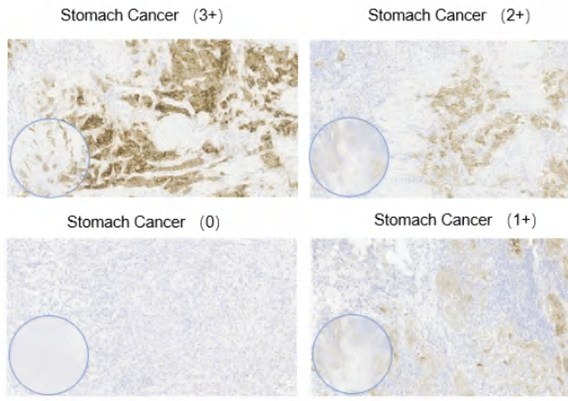
Figure 4. Claudin 18.2 expression intensity in cancer. Semi-quantitative scoring of the average tumor expression intensity (0-3+) was performed by eye. Representative images show Claudin 18.2 expression intensity observed in various pathological cancer. Magnification, x4 and x20. Image Credit: Source: ACROBiosystems
Multi-cancer staining assessment
To expand the potential beneficiary population for new drug treatments, this study conducted Claudin18.2 staining assessments on tissue samples from gastric cancer, colorectal cancer, pancreatic cancer, and ovarian cancer.
The goal was to evaluate expression across multiple tumor types and support broader clinical application of Claudin18.2-targeted therapies.
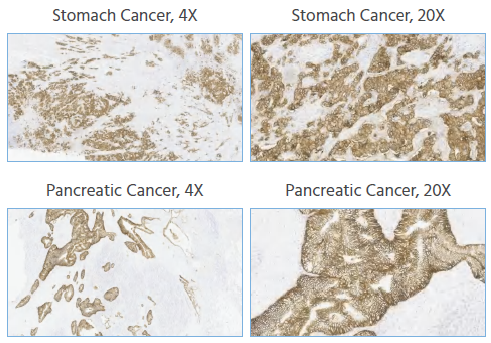
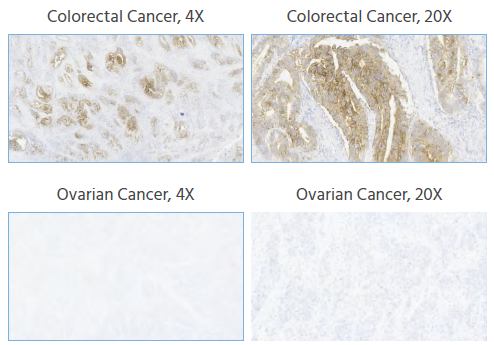
Figure 5. IHC staining of human gastric cancer, colorectal cancer, pancreatic cancer, and ovarian cancer tissues was performed using Anti-Claudin18.2 at a dilution of 1:1000, with no background staining. Magnification, x4 and x20. Image Credit: Source: ACROBiosystems
The anti-Claudin18.2 antibody demonstrated positive staining in tissue samples from gastric cancer, colorectal cancer, and pancreatic cancer, while ovarian cancer samples showed negative staining. No non-specific staining or background coloration was observed.
These results indicate that Claudin18.2 is expressed in multiple cancer types—specifically gastric, colorectal, and pancreatic—suggesting a broad potential patient population that could benefit from Claudin18.2-targeted therapies. This supports its application in screening various cancer types and in clinical diagnosis.
In this experiment, the absence of Claudin18.2 staining in ovarian cancer samples suggests that the protein is either not expressed or expressed at low levels in this tumor type. This may point to the need to expand the testing population or focus on tumor types with higher Claudin18.2 expression for clinical use.
Precision study under different experimental conditions
To prevent detection anomalies that may arise from variations in the experimental environment, precision serves as a critical measure of reliability in pathological diagnosis.
To ensure consistent and repeatable detection results, a precision study was carried out under a range of experimental conditions. Two experimenters conducted tests using Claudin18.2 primary antibodies on tissue samples across different dates, employing different immunohistochemistry instruments.
The resulting slides were then independently reviewed by different pathologists to evaluate consistency across all variables.
Inter-experimenter reproducibility
Different experimenters used the same batch of antibodies in order to test the same batch of samples.
Source: ACROBiosystems
| |
Strong Positive
Sample Count |
Weak Positive
Sample Count |
Negative
Sample Count |
| Experimenter A |
6 |
3 |
16 |
| Experimenter B |
6 |
3 |
26 |
| Precision |
100 % |
100 % |
100 % |
Inter-day reproducibility
The same experimenter conducted the same batch and sample testing on a range of different dates.
Source: ACROBiosystems
| |
Strong Positive
Sample Count |
Weak Positive
Sample Count |
Negative
Sample Count |
| Date 1 |
6 |
3 |
16 |
| Date 2 |
6 |
3 |
26 |
| Precision |
100 % |
100 % |
100 % |
Inter-equipment reproducibility
The same batch and same samples were tested on different instruments.
Source: ACROBiosystems
| |
Strong Positive
Sample Count |
Weak Positive
Sample Count |
Negative
Sample Count |
| LEICA Bond III A |
6 |
3 |
16 |
| LEICA Bond III B |
6 |
3 |
26 |
| Precision |
100 % |
100 % |
100 % |
Inter-pathologist consistency
Two pathologists independently reviewed a total of 212 tissue samples.
Source: ACROBiosystems
| |
Strong Positive
Sample Count |
Weak Positive
Sample Count |
Negative
Sample Count |
| Pathologist A |
22 |
34 |
156 |
| Pathologist B |
20 |
36 |
156 |
| Precision |
99 % |
99 % |
100 % |
Precision across different experimenters, dates, and instruments reached 100 %. Two pathologists independently reviewed 212 tissue samples, with complete agreement (100 %) on positive and negative classifications.
There were two cases of discrepancy in the degree of positivity, resulting in a 99 % concordance rate for staining intensity, which meets the accepted standard for inter-pathologist consistency and is considered acceptable.
These results demonstrate that the Claudin18.2 antibody delivers consistent and repeatable performance across varying experimental conditions. This reduces variability in testing and supports reliable participant screening and clinical diagnostic outcomes.
Conclusion
Following the development and validation of the Claudin18.2 antibody and its methodology, the data demonstrates that the Claudin18.2 immunohistochemical antibody offers strong specificity, sensitivity, and accuracy. It can clearly differentiate between Claudin18.1 and Claudin18.2, making it a valuable tool for patient selection and clinical diagnosis.
This antibody supports the development of personalized treatment strategies, contributes to improved clinical trial success rates and drug development efficiency, and helps guide treatment decisions and medication use.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
References and further reading
- Angerilli, V., et al (2024). Claudin-18.2 testing and its impact in the therapeutic management of patients with gastric and gastroesophageal adenocarcinomas: A literature review with expert opinion . Pathology - Research and Practice, 254, pp.155145–155145. https://doi.org/10.1016/j.prp.2024.155145.
- Nakayama, I., et al. (2024). Claudin 18.2 as a novel therapeutic target. Nature Reviews Clinical Oncology, 21(5), pp.354–369. https://doi.org/10.1038/s41571-024-00874-2.
- Cao, W., et al. (2022). Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomarker Research, 10(1). https://doi.org/10.1186/s40364-022-00385-1.
- Kyuno, D., et al. (2021). Claudin-18.2 as a therapeutic target in cancers: cumulative findings from basic research and clinical trials. Tissue Barriers, 10(1). https://doi.org/10.1080/21688370.2021.1967080.
- Yan, P., et al. (2024). Claudin18.2 expression and its clinicopathological feature in adenocarcinoma from various parts. Journal of Clinical Pathology, p.jcp-2023-209268. https://doi.org/10.1136/jcp-2023-209268.
- Sahin, U., et al. (2021). FAST: a randomized phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Annals of Oncology, 32(5), pp.609–619. https://doi.org/10.1016/j.annonc.2021.02.005.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.