Recently, ACROBiosystems announced its latest antigen products targeting an emergent Omicron mutation, BA.2.75.

Image Credit: ACROBiosystems
Since May, there has been an exponential spread of BA.2.75 throughout India. Shortly after this new mutation was discovered, new related cases were identified in a number of other countries around the world, including Australia, Germany, the UK and the US.
Unofficially nicknamed “Centaurus,” the World Health Organization (WHO has classified BA.2.75 as a Variant of Interest (VOIs) instead of a Variant of Concern (VOCs). Although it remains unclear what level of threat of this emerging Omicron mutation presents, medical experts around the world are concerned about its presence.
Dr. Maria Van Kerkhove, technical lead on COVID-19 at the WHO, suggested that there are an insufficient number of samples for a comprehensive analysis of BA.2.75. Currently, only 200 sequences have been taken from 14 countries: a significant limiting factor when it comes to better understanding this mutation.
Due to the fact a large portion of the population is immunized against COVID-19, newer variants are demonstrating a high level of immune evasion, which is also becoming the norm. As such, infection rates by novel COVID variants are expected to rise in spite of a significant number of people being vaccinated.
Such concerns were similarly confirmed in a recent study by Yamasoba, et al. Out of 10 therapeutic monoclonal antibodies tested against BA.2.75 and other novel variants, the majority of therapies approved failed to neutralize the COVID variants, particularly BA.2.75.2
Contrary to the other Omicron subvariants, Centaurus (BA.2.75) has shown to display additional mutations beyond the same high-level variant structure as Omicron BA.2. There are 17 more nucleotide mutations of BA.2.75 in contrast to BA.2 as confirmed by recent sequence analysis of both variants.v
Numerous key mutations, G446S and Q493R on the Spike protein, have considerable antigen-converting effects. Based on studies previously conducted, the G446S mutation is one of the most efficient immune escape sites, while the Q493R mutation may consolidate the affinity between the virus and the ACE2 receptor.
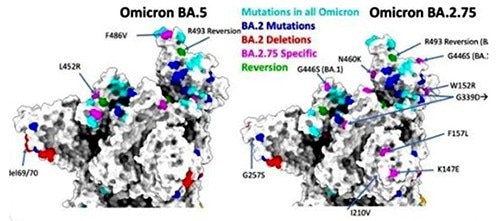
Image Credit: ACROBiosystems
The emergence of novel COVID variants with greater rates of infection places a spotlight on the long-term vaccine strategy and raises many questions.
An increasing number of experts have begun to adopt the idea of utilizing a ‘booster shot’ rather than continuing to develop new vaccine programs to combat the new variants. Recently, Moderna announced that two potential boosters targeting the Omicron subvariants are expected to be released this fall.
![Neutralization neutralization assays were performed performed using pseudoviruses harboring the SARS-CoV-2 Spike proteins of BA.2, BA.4/5, and BA.2.75. YAMASOBA .[2]](https://www.news-medical.net/images/appnotes/ImageForAppNote_4147_16606491806819903.jpg)
Neutralization assays were performed using pseudoviruses harboring the SARS-CoV-2 Spike proteins of BA.2, BA.4/5, and BA.2.75. YAMASOBA.2. Image Credit: ACROBiosystems
In today’s COVID-19 landscape, it remains that the dominant coronavirus strain in a number of countries is the extremely contagious BA.5 Omicron subvariant. As reported by the Global Initiative on Sharing All Influenza Data (GISAID), the data surrounding BA.5 accounted for 52% of the sequence library.
Moreover, the study highlights that the R0 value, a nominal value that indicates infectiousness, is 18.6. This is around six times more infectious than the wild-type, making it the most transmissible subvariant of the SARS-CoV-2 virus.
To support the development of vaccines and respond to the rapid demands of the market, i.e., targeting newer and emerging COVID variants, ACROBiosystems’ has released a new range of BA.2.75 antigen-related products.
With the changing nature of COVID-19, ACROBiosystems continuously strives to supply the evolving key antigens for vaccine research, including the Spike timer, S RBD, and Nucleocapsid protein.
Main features
- Availability of Spike trimer, S RBD, S1, S2, NTD and N protein in biotinylated and unconjugated versions
- Encompasses almost all new Omicron subvariants: BA.4/5, BA.2.74/75/76, BA.2.38, etc.
- High bioactivity is validated by stringent quality control processes, which are appropriate for ELISA/SPR/BLI and other experiments
- Purity exceeds 95% verified by SDS-PAGE and over 90% determined by MALS
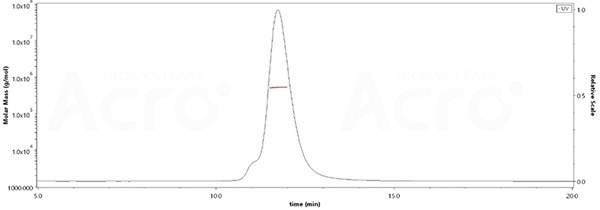
The purity of SARS-CoV-2 Spike Trimer, His Tag (BA.2.75/Omicron) (Cat. No. SPN-C522f) is more than 90% verified by SEC-MALS. The molecular weight of this protein is around 496-548 kDa. Image Credit: ACROBiosystems
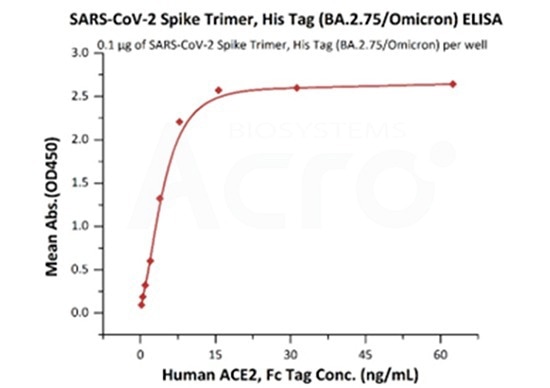
Measured by its binding ability in a functional ELISA. Immobilized SARS-CoV-2 Spike Trimer, His Tag (BA.2.75/Omicron) (Cat. No. SPN-C522f) at 1 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.2-16 ng/mL (QC tested). Image Credit: ACROBiosystems
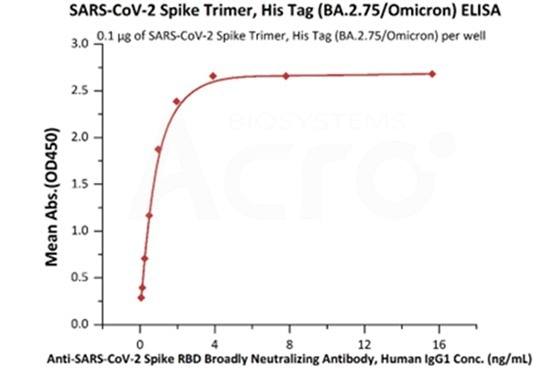
SARS-CoV-2 Spike Trimer, His Tag (BA.2.75/Omicron) (Cat. No. SPN-C522f) at 1 μg/mL (100 μL/well) can bind Anti-SARS-CoV-2 Spike RBD Broadly Neutralizing Antibody, Human IgG1 (Cat. No. SPD-M265) with a linear range of 0.1-2 ng/mL (Routinely tested). Image Credit: ACROBiosystems
References
- Topol, E., 2022. BA.5, Chapter 2. [online] Erictopol.substack.com. Available at: https://erictopol.substack.com/p/ba5-chapter-2.
- Yamasoba D, Sato K, et al, 2022. Neutralization sensitivity of Omicron BA.2.75 to therapeutic monoclonal antibodies. The Lancet Infectious Diseases.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.