Precision-targeted therapy via antibody drugs has become central to the field of cancer immunotherapy. Monoclonal antibodies (mAbs) have historically played a key role in targeted therapy, but mAbs face several limitations in terms of their ability to address the complex tumor microenvironment.
Researchers are currently exploring more advanced antibody designs to overcome these challenges, with multispecific antibodies (MsAbs) emerging as a major breakthrough in this area and evolving into a key research area in cancer immunotherapy research.
From monoclonal to multispecific: The importance of complex antibodies
mAbs have seen considerable success in treating diseases such as autoimmune disorders and cancer, but their single-target mechanism of action has limited their effectiveness in treating complex diseases.
Cancer cells in the tumor microenvironment typically leverage multiple mechanisms to evade immune detection, making it challenging for a single antibody to provide a comprehensive anti-tumor effect.
Researchers have developed bispecific antibodies (BsAbs) to overcome this limitation. These antibodies integrate two antigen-binding sites within a single molecule to improve antitumor activity.
Advances in technology have enabled the development of antibody drugs able to simultaneously target three or more antigens. This work represents a promising breakthrough in cancer treatment, and has led to the development of MsAbs, including tetraspecific antibodies (TetraMabs) and trispecific antibodies (TsAbs).
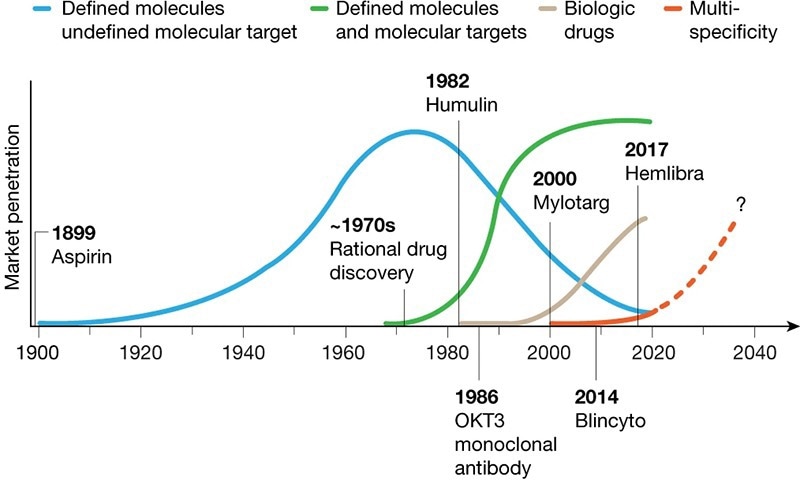
Four Transformative Waves Have Shaped the Development of the Biopharmaceutical Industry. Image Credit: https://doi.org/10.1038/s41586-020-2168-1
Mechanism of action of MsAbs
BsAbs: Dual attack, precise killing
BsAbs improve targeting ability and enhance therapeutic efficacy through the simultaneous binding of two different targets. The CD3×TAA (tumor-associated antigen) T-cell engager (TCE) is one of the most common BsAb designs.
TCE offers the ability to directly recruit T-cells to the tumor microenvironment, activating these to release cytotoxic factors such as Blincyto (CD3×CD19) that can kill the tumor.
Dual TAA targeting is another useful strategy that enhances tumor specificity by recognizing two tumor antigens. This approach reduces the risk of resistance triggered by antigen loss, for example, Zanidatamab (HER2×HER2).
Some BsAbs also combine tumor antigens with immune checkpoints like CD47×PD-L1, offering both immunoregulatory and antitumor effects.
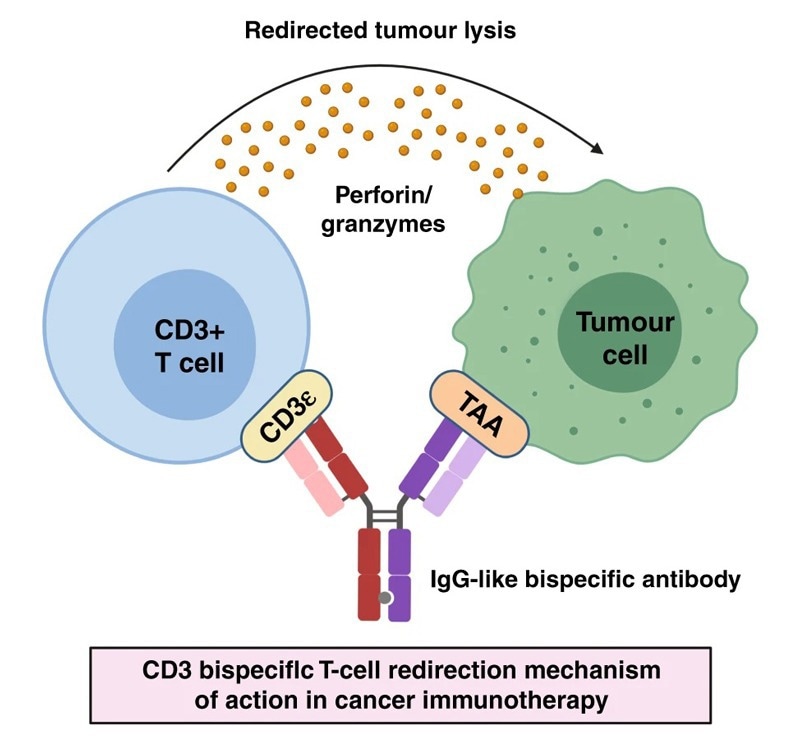
Mechanism of CD3 BsAbs-mediated T-cell redirection in cancer. Image Credit: https://doi.org/10.1038/s41416-020-01225-5
TsAbs: Multidimensional synergy, overcoming resistance
TsAbs expand on BsAbs’ design, adding a third target to improve immune effects or overcome resistance. Certain TsAbs combine CD3 with two different TAAs in order to reduce the risk of antigen loss and improve tumor specificity.
For instance, Simcere Pharmaceutical’s SIM0500 targets BCMA, GPRC5D, and CD3, effectively overcoming resistance, killing multiple myeloma cells, and maintaining long-term tumor suppression.
Another popular strategy is the introduction of costimulatory receptors, such as Sanofi’s SAR442257 (CD38/CD3/CD28). This antibody mediates T-cell killing of CD38-positive tumors via CD3, improving T-cell activation via the CD28 costimulatory signal.
Numab Therapeutics AG also developed NM32-2668 to extend the treatment’s half-life. This antibody targets CD3, ROR1, and human serum albumin (HSA), redirecting T-cells to attack ROR1-positive tumors, optimizing the dosing regimen by extending circulation time via its HSA-binding domain.
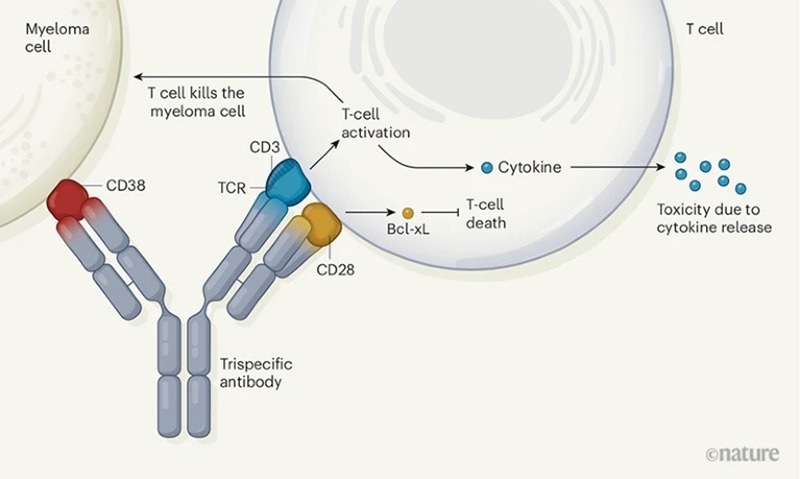
TsAbs Targeting CD38/CD3/CD28. Image Credit: https://doi.org/10.1038/d41586-019-03495-3
TetraMAbs: Comprehensive regulation, expanding immune killing potential
TetraMAbs further mitigate targeting limitations, facilitating a more wide-ranging anti-tumor regulation strategy. Biokin Pharmaceutical has developed a number of TetraMAbs, including GNC-038 (CD3×4-1BB×PD-L1×CD19).
GNC-038 amplifies anti-tumor immune effects via a quadruple mechanism: T-cell activation, immune checkpoint blockade, costimulation enhancement, and tumor-specific targeting.
This multidimensional regulation enhances efficacy and reduces the risk of tumor immune escape. These capabilities represent a more powerful approach to cancer immunotherapy.
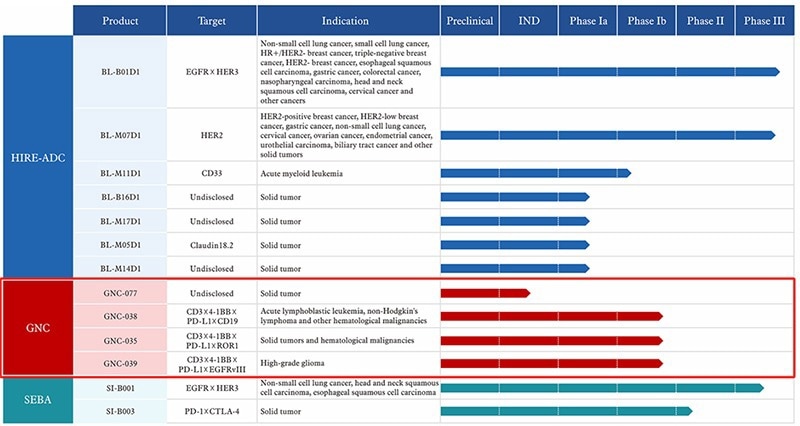
Some of Biokin Pharmaceutical's Pipelines. Image Credit: Biokin Pharmaceutical Official Website
Conclusion
Developing antibody drugs such as mAbs and MsAbs has significantly improved the efficacy and precision of cancer immunotherapy.
BsAbs offer the ability to increase the robustness of tumor-killing ability via a dual-target mechanism, TsAbs overcome resistance and optimize immune effects, and TetraMAbs expand immune killing potential via comprehensive regulation.
These innovative strategies enhance antibody drugs’ efficacy and represent increasingly promising solutions for cancer treatment. As antibody engineering technology continues to evolve, it is anticipated that MsAbs will play an even greater clinical role in the future.
ACROBiosystems offers a comprehensive portfolio of high-quality CD3 proteins, including CD3E&CD3G, CD3E&CD3D, CD3D, CD3E, and CD3G.
CD3E&CD3G and CD3E&CD3D have been verified as a 1:1 heterodimer using MALS and nonreductive electrophoresis.
CD3 proteins have also been extensively tested for binding activity with different TCEs (for example, CD3×BCMA and CD3×DLL3) and clinical-stage bispecific antibodies (for example, SP34-2, OKT3, and UCHT1), supporting antibody drug screening and optimization.
References and further reading
- Deshaies, R.J. (2020). Multispecific drugs herald a new era of biopharmaceutical innovation. Nature, (online) 580(7803), pp.329–338. https://doi.org/10.1038/s41586-020-2168-1.
- Singh, A., Dees, S. and Grewal, I.S. (2021). Overcoming the challenges associated with CD3+ T-cell redirection in cancer. British Journal of Cancer, (online) 124(6), pp.1037–1048. https://doi.org/10.1038/s41416-020-01225-5.
- Garfall, A.L. and June, C.H. (2019). Trispecific antibodies offer a third way forward for anticancer immunotherapy. Nature, (online) 575(7783), pp.450–451. https://doi.org/10.1038/d41586-019-03495-3.
- Goebeler, M.-E., Stuhler, G. and Bargou, R. (2024). Bispecific and multispecific antibodies in oncology: opportunities and challenges. Nature Reviews Clinical Oncology, (online) pp.1–22. https://doi.org/10.1038/s41571-024-00905-y.
Acknowledgements
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.