
Image Credit: ACROBiosystems
Neurogenerative diseases are a significant burden on the global health system at the personal, public health, and societal levels. Degenerative, progressive neurological disorders are increasing in prevalence. They include Parkinson’s Disease (PD) and Alzheimer’s Disease (AD.)
Currently incurable, over 130 million people globally are afflicted with either AD or PD. The prevalence of these diseases increases dramatically with age. The aging world population is causing increased burdens on the global health system due to the increasing prevalence of neurogenerative disorders.
As life spans continue to increase, the number of patients afflicted with these disorders is predicted to increase in the near future. While this is a growing public health emergency, neurological disease research is hindered by inadequate understanding or incomplete perspectives on disease pathology.
This has translated to a woefully poor track record in developing efficacious treatments for neurodegenerative disease and discovering new therapeutic avenues.
Using in vivo and in vitro models has been key in elucidating disease pathology. Both these models are invaluable tools that provide new insights into cellular mechanisms, disease progression, and several other vital factors that influence the development and severity of numerous medical conditions.
In regard to neurodegenerative disease, limited comprehension is partly due to the use of premature experimental models.1 Transgenic mouse models are typically used in existing models, employing over-expressed or knock-out proteins and pathogenic genes.
These types of models have some key drawbacks, however, as they do not accurately and completely reproduce natural disease progression. Therefore, they provide an incomplete imitation of neurodegeneration in human patients.
Pre-formed fibrils (PFFs) are a recently developed novel tool. They can model PD and AD, and are increasingly being employed in related studies in neurodegenerative disease research.
PFFs are generated in vitro and can effectively imitate endogenous protein aggregation in cellular and animal models. More accurate models which closely align with neurogenerative disease traits, including transmission and seeding of pathogenic proteins, can be enabled using pre-formed fibrils.
In this article, the current limitations of conventional disease models are discussed, along with an in-depth discussion about PFFs and their current applications in neurogenerative disease research.
Current neurodegenerative disease models
Protein misfolding and aggregation is a key characteristic of neurogenerative diseases. This results in cellular dysfunction, synaptic connection loss, and, ultimately, damage to the brain.
While the proteins involved differ between different diseases, clinical indications and disease progression are similar.
Most of the current experimental approaches used to study neurodegenerative disease are based on animal models, with transgenic rodents widely used in research that express human genes, especially for AD research
Other models involve physically and chemically induced animals as these models can capture disease symptoms that transgenic animal models struggle with, such as PD.
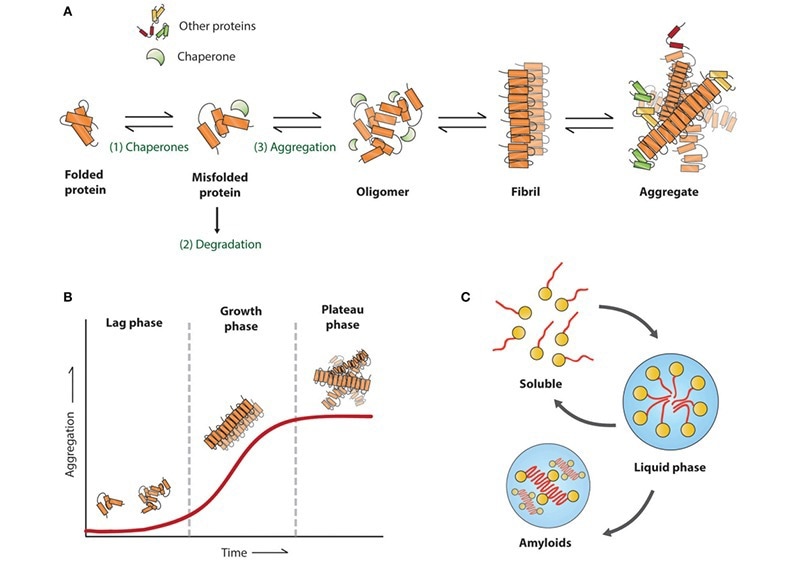
Figure 1. Amyloid aggregation associated with neurodegenerative diseases. Reproduced from Stroo et al.2 Image Credit: ACROBiosystems
Transgenic mouse models typically employed in AD research only exhibit amyloid accumulation, a key characteristic that defines AD. However, they lack neurofibrillary tangle (NFT) development. These insoluble, twisted fibers in the brain are a secondary characteristic of AD.
A misfolded tau protein (a constituent of microtubule structures) leads to the formation of NFTs. Microtubules transport nutrients and other molecules between nerve cells in the brain. When NFTs form, these microtubule structures collapse.
In transgenic mouse models, only 4R tau isoforms are expressed due to endogenous mouse tau inhibiting human tau aggregation. 3R and 4R isoforms are present in Alzheimer’s Disease.
Transgenic mice can be modified using a P301S or P301L mutation to express 4R human tau, overcoming the issue of NFT non-formation. However, this reduces model accuracy due to these mutations not being associated with AD. Furthermore, this can influence toxicity or cause interaction with amyloid plaques.
Overexpression of mutated tau proteins in transgenic mouse models can result in motor deficits typically not associated with Alzheimer’s Disease. Furthermore, the accuracy of cognitive testing can be impacted by this strategy.3
The classical, widely used approaches for modeling PD are toxin-induced mouse models. These include MPTP and 6-OHDA. Neurotoxins are introduced into animals used in these models, inducing dopaminergic neuron degeneration in PD-related regions. This is a rapid degeneration.
Using this approach produces a robust and accurately characterized motor deficit. However, only the clinical symptoms are stimulated in these models. The molecular pathology of PD cannot be accurately replicated in this approach. Types of PD-associated molecular pathology include Lewes bodies and α-syn accumulation.
Transgenic mice with specific mutations in DJ-1, PINK1, Parkin, SNCA, and LRRK2 are also used in PD research. Linked to inherited PD forms, these mutations express PD-implicated proteins. However, these models often lack dopamine neuron loss. They lack the consistent, reproducible deficits that toxin-induced models display.
Pre-formed fibrils and formation
Pre-formed fibrils are more cost-effective for neurodegenerative disease research than transgenic and toxin-induced models and provide a different perspective PFFS overcome the reported issues with conventional transgenic and toxin-induced mouse models.
Employing PFFs in studies is more direct, with pre-formed pathogenic fibrils being injected. Protein aggregates are formed, inducing rapid neurodegeneration. The scientific burden and length of mouse maturation time are reduced, and molecular pathology is induced more rapidly than in transgenic mouse models.
In addition, PFFs can be used at the in vitro level. This innovative approach enables a direct method with improved reproducibility. Thus, enhanced identification and measurement of disease pathology and a model for testing new drug candidates are promoted by employing pre-formed fibrils.
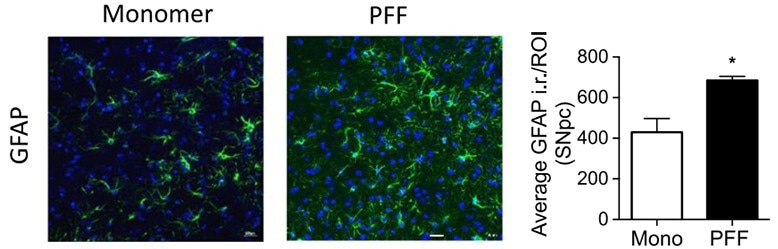
Figure 2. Immunohistochemical GFAP staining of the substantia nigra pars compacta region in a mouse brain. Optical density analysis of GFAP-positive cells reveals an increased amount of astrogliosis in PFF α-syn treated mice in comparison to monomer α-syn, revealing neuroinflammation associated with α-syn accumulation. Reproduced from Earls 2019.4 Image Credit: ACROBiosystems
PFFs are formed in a simple process at room temperature from protein monomers. Monomers are incubated and shaken either with or without heparin. The ability of the monomer of choice to aggregate influences the selection protocol. Different PFFs require different concentrations and incubation periods.
For instance, tau-441 PFFs require an initial monomer concentration of 2 mg ML-1 in the presence of heparin and a seven-day incubation and shaking period. Alpha-synuclein PFFS, in contrast, requires an initial monomer concentration of 5 mg mL-1 and does not require heparin.
Other monomers and pathogenic fragments with increased aggregation tendencies require a shorter incubation period (typically 4-5 days.)
Before their use as disease models, PFF verification is a key consideration. Visual and chemical verification should be employed to verify the presence of fibrillary structures. ThT assays can be employed for chemical verification, as they are useful for analyzing high molecular weight species.
ThT assays are also effective at determining the presence of adequately formed cross-beta structures in prepared pre-formed fibrils. TEM or AFM should be employed to confirm aggregate size and morphology. Moreover, PFFs should be sonicated before use in seeds under 50 nm due to the importance of fibril length in pathogenicity.
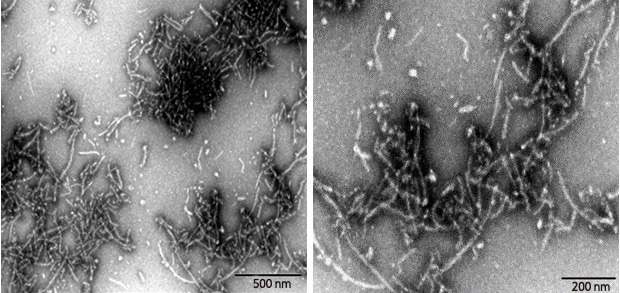
Figure 3. Transmission Electron Microscopy of Human Tau-441/2N4R pre-formed fibrils(ACROBiosystems, Cat. No. #TAU-H5115). Protein aggregates with distinct fibrous structures are visible with accurate morphology. Image Credit: ACROBiosystems
Current applications of pre-formed fibrils
Research has developed several well-established tau PFF models for use in neurons, astrocytes, microglia, and other neurological cellular systems. These have been employed successfully in studying neurodegenerative diseases, such as AD, and drug discovery and development.
Studies on PFFs have demonstrated that tau PFFs undergo internalization by cells in the brain by endocytosis. They can act as a seed for endogenous tau and induce misfolding.5,6 After internalization, the mouse’s endogenous tau undergoes intracellular fibrilization.
Tau aggregates are externalized after recruitment via mechanisms such as degenerating axions. Unknown mechanisms may be involved in this process. Interconnected and surrounding neurons then adopt these externalized tau aggregates.
Animal-based tau pre-formed fibril models have been more challenging to establish than cell-based models. Injection of some types of tau PFFs appears to have limited seeding capacity.
One study used in vitro approaches to introduce truncated tau PFFs into transgenic mice through the frontal or hippocampal cortex. Tau fibrilization and the spread of pathology to the interconnected region of the brain were observed through a combination of PFF models and transgenic mice.
The results of this study demonstrated hippocampal neuronal loss. The study’s observations led to a new in vitro model for neurodegeneration and pathological spread.
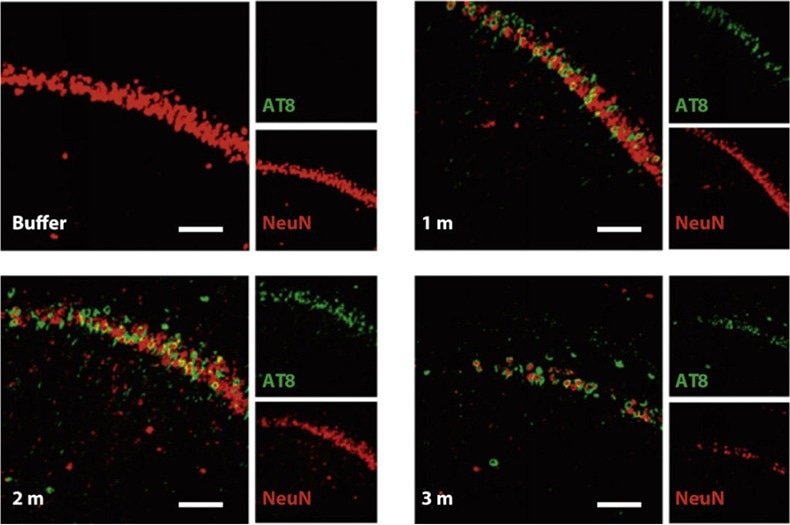
Figure 4. Nissl staining of the hippocampus of P301L mice injected with buffer or truncated tau PFFs. AT8 (green, identifier of tau-presence) was apparent in mice treated with PFFs and shown to preceed neuronal loss found in the 3 month post-injection mice. Reproduced from Peeraer 2015.7 Image Credit: ACROBiosystems
α-syn PFF models have widespread use in PD research. These models are used to model two major disease processes in PD progression: selective midbrain dopamine neuron degeneration and intraneuronal Lewy body/neurite accumulation.
Tau PFFs cannot properly induce protein aggregates in vivo, but α-syn PFF models have been extensively established in both in vivo and in vitro. In-vitro models have been used to target organ-on-a-chip10, human-iPSC-derived dopaminergic neurons9, and primary neurons8 in research.
These methods are highly suitable tools for high-throughput drug screening and testing. Other studies have demonstrated the effectiveness of α-syn PFF seed aggregation and fibrilization in wild-type mice.
One study injected α-syn PFFs which targeted the dorsal striatum in mice. This approach successfully identified hyperphosphorylated α-syn deposits.11 30 days post-injection, Lewy-body-like accumulations were observed in the inter-neural connectivity. This suggested cell-to-cell transmission methods.
The study also observed PD-related clinical symptoms. Injecting a single misfolded α-syn PFF initiated the PD-associated neurodegenerative cascade.
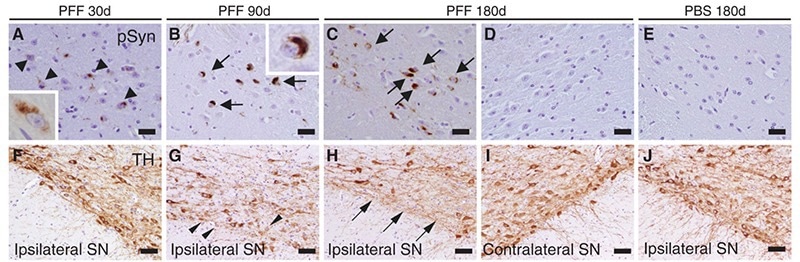
Figure 5. α-Syn immunostaining of substantia nigra pars compacta of mice following intrastriatal PFF injection. Lewy-body like inclusions (black arrows) were observed in ipsilateral sections but were not observed in contralateral sections; this supports the theory of a cell-to-cell transmission method for Lewy-bodies comprised of misfolded α-syn. Reproduced from Luk 2012.11 Image Credit: ACROBiosystems
Conclusion
Novel tools that can model more “natural” neurodegenerative disease pathophysiology, pre-formed fibrils have demonstrated efficaciousness in disease progression modeling and drug discovery and development. They have been used to study both PD and AD, in both in-vivo and in-vitro studies.
PFFs can accurately mimic pathogenic protein seeding and transmission activity. They are more suitable than toxic-induced and transgenic mouse models, providing enhanced neurodegenerative disease hallmark replication with reduced scientific burdens.
While they have proven effective in academic studies, clinical research using PFFs is still limited. Reproducible protocols and quantifiable, stable phenotypes are needed to widen the large-scale drug screening and testing applications of pre-formed fibrils.
While key challenges persist in research, many researchers and companies are focusing on developing PFF models by analyzing their activity mechanisms and structural features. A deeper understanding will enhance the development of high-quality PFFs for neurological disease research.
If these challenges are addressed properly in future research, PFFs could represent a revolutionary breakthrough in providing clinical researchers with an advanced neurogenerative disease therapeutic modeling platform.
References and Further Reading
- Gitler AD, et al. (2017) Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. 10(5):499-502.
- Stroo E, et al. (2017). Cellular Regulation of Amyloid Formation in Aging and Disease. Front Neurosci. 14;11:64.
- Drummond E & Wisniewski T. (2017) Alzheimer's disease: experimental models and reality. Acta Neuropathol. 133(2):155-175.
- Earls, R.H., et al. (2019) Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J Neuroinflammation. 16, 250.
- Frost B, et al. (2009) Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284:12845–12852.
- Guo JL & Lee VM. (2011) Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 286:15317–15331.
- Peeraer E, et al. (2015) Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol Dis. 73:83-95.
- Volpicelli-Daley LA, et al. (2011) Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 72(1):57-71.
- Tanudjojo, B., et al. (2021) Phenotypic manifestation of α-synuclein strains derived from Parkinson’s disease and multiple system atrophy in human dopaminergic neurons. Nat Commun. 12, 3817.
- Pediaditakis I, et al. (2021) Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nat Commun. 12(1):5907.
- Luk KC, et al. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 338(6109):949-53.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.