Biosimilars are biological drugs that are of high similarity to other biological drugs that have already been approved, they are also called reference innovator products.
Biosimilars must be thoroughly tested throughout their development process in order to prove that potential differences between the biosimilar and its reference are not clinically significant in terms of efficacy (EMA)/potency (FDA), safety, quality and purity.
The binding that occurs between a biosimilar and its target antigen and Fc receptors is used as a key measurement of its predicted efficacy. ACROBiosystems has developed Fc receptor proteins and target antigen proteins of high quality to assist in research involving biosimilars.
Product List
| Target Antigen Proteins: |
| Her2 |
TNF-alpha |
EGF R |
VEGF165 |
VEGF121 |
CD20 |
| RANKL |
PD-1 |
PD-L1 |
CTLA4 |
|
|
| Fc Receptor Proteins: |
| FcRn |
Fc gamma RIIIA / CD16a |
Fc gamma RIIIB / CD16b |
Fc gamma RIIA / CD32a |
Fc gamma RIIB / CD32b |
Fc gamma RI / CD64 |
Why Choose ACROBiosystems?
Validated with Reference Innovator Drugs
The majority of Fc receptors and target antigens that are provided by ACROBiosystems have been validated against their reference using ELISA, SPR or BLI assays.
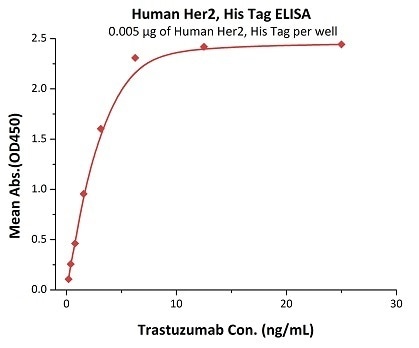
Figure 1. Immobilized Human Her2, His Tag (Cat. No. HE2-H5225) at 0.05 μg/mL (100 μL/well) can bind Trastuzumab with a linear range of 0.2-3 ng/mL.
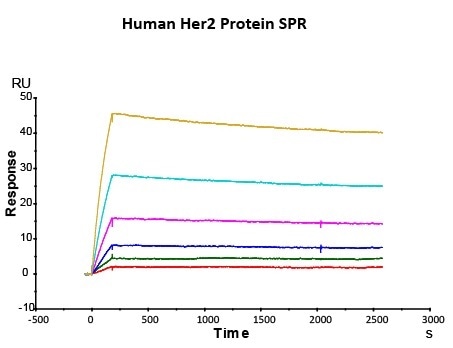
Figure 2. Immobilized trastuzumab on CM5 Chip via anti-human Fc IgG, can bind Human Her2 Protein (Cat. No. HE2-H5225) with an affinity constant of 0.147nM as determined in SPR assay (Biacore T200).
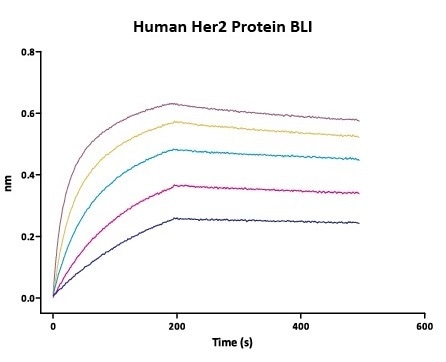
Figure 3. Immobilized trastuzumab on AHC Biosensor, can bind Human Her2 Protein (Cat. No. HE2-H5225) with an affinity constant of 0.93 nM as determined in BLI assay (Fortebio Octet 96).
High Batch-to-Batch Consistency
ACROBiosystems always use thorough quality control processes to guarantee that their products show high consistency between batches.
Newly produced products are subjected to side-by-side comparison with the internal standard in a variety of assays. Only those within an acceptable margin of difference are allowed to be released.
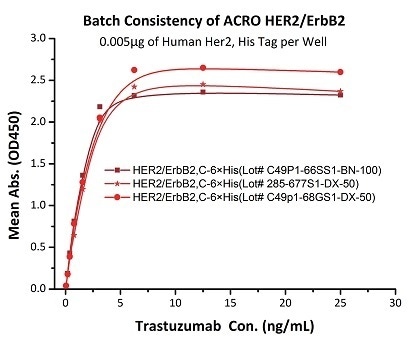
Figure 4. Binding activity of three different lots of hHER2 (Cat. No. HE2-H5225) were evaluated in the above ELISA analysis against Trastuzumab. The result showed that the batch variation among the tested samples is negligible.
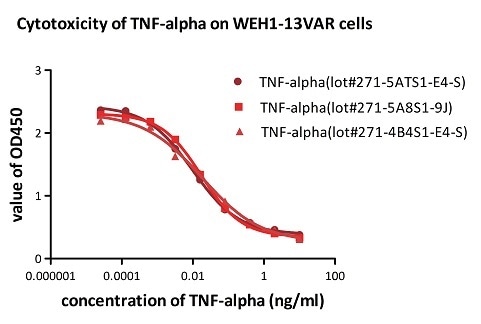
Figure 5. Recombinant Human TNF-alpha (Cat. No. TNA-H4211) induces cytotoxicity effect on the WEH1-13VAR cells in the presence of the metabolic inhibitor actinomycin D. The ED50 for this effect is 0.007-0.014ng/ml. The result shows that the batch variation among the tested samples is negligible.
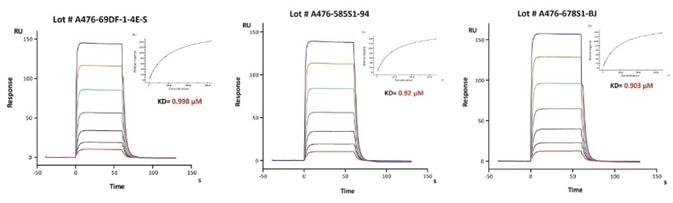
Figure 6. Immobilized trastuzumab on CM5 Chip, can bind Human FcRn / FCGRT & B2M Heterodimer Protein (Cat. No. FCM-H5286) with an affinity constant of about 0.9 μM as determined in SPR assay (Biacore 8K). The result shows that the batch variation among the tested different lots is negligible.
High Stability and High Activity
ACROBiosystems carries out both accelerated and long-term stability testing in order to guarantee protein stability and ensure the product lifetime is correct. The proteins remain fully intact throughout the testing process, meaning quality is retained and there is no loss in activity.
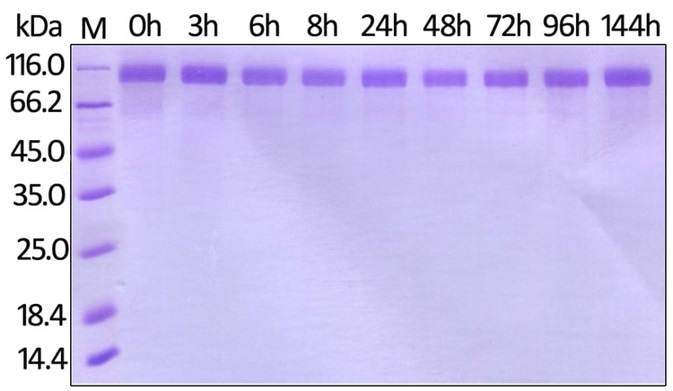
Figure 7. Human Her2, His Tag (Cat. No. HE2-H822R) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue.
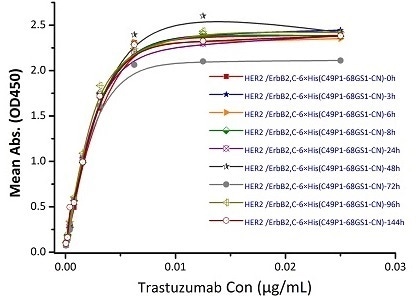
Figure 8. Immobilized Biotinylated Human Her2, His Tag (Cat. No. HE2-H822R) at 0.05 μg/mL (100 μL/well) can bind Trastuzumab. The result shows that the Biotinylated Human Her2, His Tag (Cat. No. HE2-H822R) is stable at 37℃ for 144 hours without performance reduction.
High Purity and High Quality
To meet the high purity requirement of pharmaceutical applications, a majority of the proteins from AcroBiosystems have to go through both SDS-PAGE and HPLC analyses. Only those meeting all requirements will be issued a lot-specific certificate of assurance and be released.
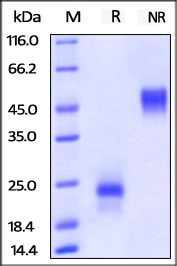
Figure 9. ActiveMax® Human VEGF165 (Cat. No. VE5-H4210) on SDS-PAGE under reducing (R) and no-reducing (NR) conditions. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 98%.
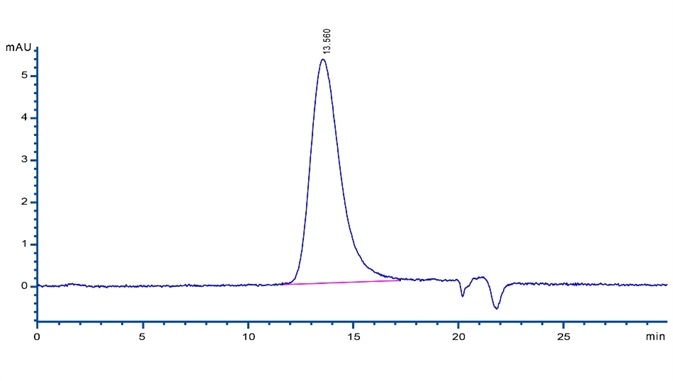
Figure 10. A SEC-HPLC analysis showing 95% of ActiveMax® Human VEGF165 (Cat. No. VE5-H4210) present as active homodimers.
References
- European Medicines Agency. Guideline on Similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1), 2014 [accessed 15.05.15]
- U.S. Department of Health and Human Services. Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Available at: (last accessed 10 November 2015)
- Bui, L.A. et al. Key considerations in the preclinical development of biosimilars, Drug Discov Today (2015)
- Al-Sabbagh A. et al. Development of biosimilars, Seminars in Arthritis and Rheumatism (2016)
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.