T Cell Engagers (TCEs) are bispecific antibodies that target both tumor-associated cell surface antigens (TAA) and T-cell surface receptors (TCR).
TCE bispecific antibodies have a major advantage over typical monoclonal antibodies due to their potent targeted cytotoxic activity. TCEs can stimulate greater and longer-lasting immunological responses by attracting and activating T cells directly.
In recent years, many companies have pushed the commercialization of TCE bispecific antibodies, seeing them as a new growth sector following ADCs.
By the end of 2024 there were roughly 400+ TCE bispecific antibody drug pipelines under development worldwide, and the number of pipelines continues to grow.
Over half of these pipelines target non-solid tumors (such as multiple myeloma and various types of lymphoma) and hematological diseases, an approximately 50% of that half are entering clinical trials.
TCE bispecific antibodies targeting solid tumors account for around 30% of the remaining pipelines.
In May 2024, Amgen reported its TCE antibody, Tarlatamab (brand name IMDELLTRA), which targets small cell lung cancer has achieved FDA accelerated approval for market release.
Mechanism of action for TCE drug
TCEs are a novel form of antibody with distinctive Fab and Fc structures. Their mechanism of action involves one Fab binding to a specific tumor-associated antigen (TAA), and the other Fab attaching to the T-cell receptor (TCR). CD3 is a common target for binding.
TCEs use dual targeting to precisely detect tumor cells while also attracting T cells to the tumor cell surface. This activates T cells and induces tumor cell death.
Activated T cells form an immunological synapse with tumor cells, resulting in tumor cell lysis and the release of cytokines (e.g., IFN-γ, TNF-α, IL-2, IL-10), which boost T cell proliferation and antitumor effects (Figure 1).
In addition, the Fc region of TCE can significantly extend the drug’s half-life and decrease its metabolism, increasing the durability of its overall therapeutic effects.
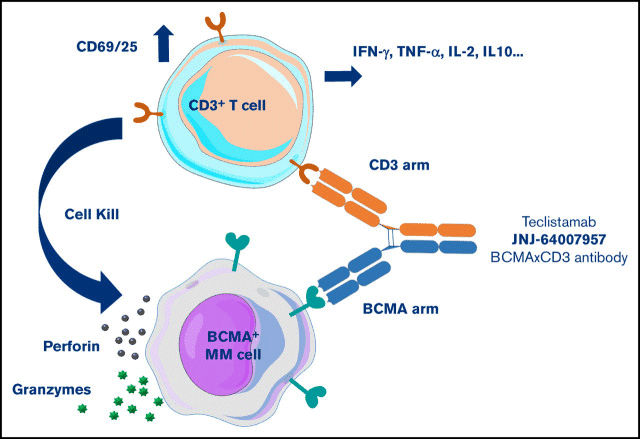
Figure 1. Schematic of the TCE Bispecific Antibody Mechanism. Image Credit: ACROBiosystems
TCE bispecific antibodies offer substantial advantages over standard IgG monoclonal antibodies.
Traditional IgG monoclonal antibodies stimulate antigen-presenting cells via Fc-mediated processes such as ADCC, CDC, and ADCP, which then activate T cells.
This indirectly inhibits the capacity of monoclonal antibodies to directly activate T cells and kill cancer cells.
TCE bispecific antibodies, on the other hand, are uninhibited and can directly activate T cells, dramatically improving their anticancer activity.
A particularly useful aspect of TCE drugs is that the Fab region can be tailored to target specific TAAs in various tumor types.
Popular targets for hematological cancers include CD19, CD20, and BCMA, and common solid tumor targets include DLL3, HER2, and CLDN18.2.
Cytokine detection in TCE drug development
During early drug development and preclinical development, researchers use in vitro cultured lymphocytes to induce cytokine release and assess the efficiency of TCE medicines on immune cells.
For the TCE bispecific antibody Tarlatamab, which targets CD3 and the small cell lung cancer-specific antigen DLL3, the mechanism of action was established by detecting the secretion of cytokines such as IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the cell supernatant at different drug concentrations (Figure 2).
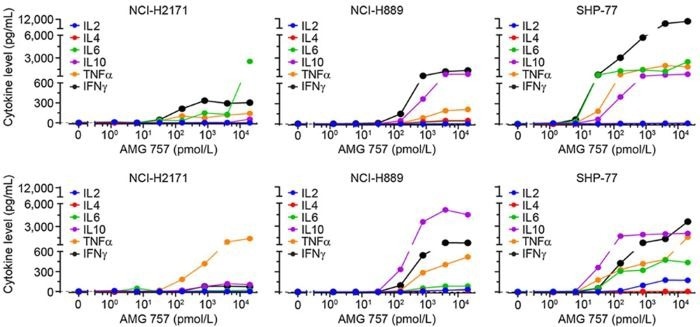
Figure 2. In Vitro Cell Stimulation Experiment of Tarlatamab. Image Credit: ACROBiosystems
Blinatumomab, a TCE medication authorized in 2014, also targets T-cell surface CD3 and CD19, a major B-cell lymphoma-specific antigen.
The FDA has identified several cytokines (IL-2, IFN-γ, TNF-α, IL-4, IL-6, IL-8, IL-10, IL-12) as biomarkers for T-cell activation in clinical-phase pharmacodynamics (Figure 3).
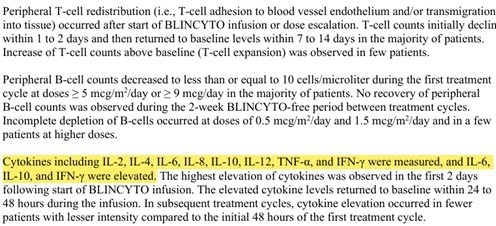
Figure 3. Cytokine Biomarker Detection in Blinatumomab Clinical Trials. Image Credit: ACROBiosystems
Summary
Cytokines are important detection indicators in the development of TCE bispecific antibody therapeutics.
By detecting the release levels of various cytokines in cell supernatants or serum during early pharmacological investigations, preclinical and clinical trials the activation status of different immune systems can be revealed.
ACROBiosystems provides a variety of quantitative ELISA kits for cytokines and biomarkers to help accelerate TCE medication development.
The ACROBiosystems kits are rigorously tested using real samples and experiments to ensure that the analytical results collected are precise, specific, accurate, sensitive, and consistent.
Case study
In Vitro activity evaluation of TCE drug blinatumomab
Experimental protocol
- Use Human PBMC cells as effector cells and GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads (ACROBiosystems, Cat. No. GMP-MBS001) to expand T cells.
- On Day 10, FACS-sort CD3+ T cells and co-culture them with Raji cells. Then, stimulate with various concentrations of TCE bispecific antibody (Blinatumomab).
- The TCE bispecific antibody binds to both TCR/CD3 on effector cells and tumor antigens on target cells.
- TCE binding leads to the release of IFN-γ, IL-6, and IL-10. Collect cell supernatants at 0, 24, and 48 hours and use ClinMax™ Human Cytokines ELISA Kit to evaluate cytokine levels.
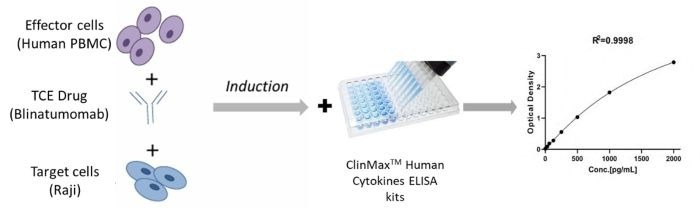
Figure 4. Assay Protocol for Measuring CD3 Bispecific Antibody (Blinatumomab) Activity. Image Credit: ACROBiosystems
Results
Co-cultured effector and target cells at an E:T ratio of 1:10 or 1:5 leads to increased IFN-γ release with drug concentration. IFN-γ secretion was highest at a 100 ng/mL concentration of Blinatumomab. (Figure 5).
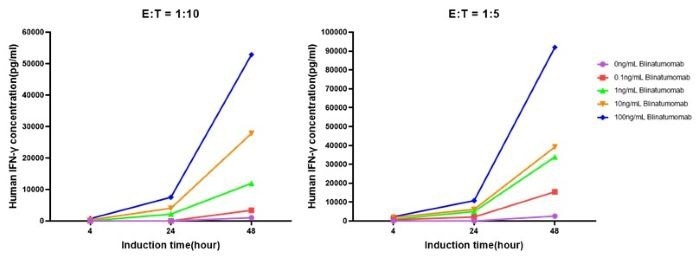
Figure 5. Elevation of IFN-γ under Different Stimulation Conditions. Image Credit: ACROBiosystems
When effector and target cells are co-cultured at E:T ratios of 1:10 and 1:5, IL-6 and IL-10 production increase with drug concentration. (Figure 6).
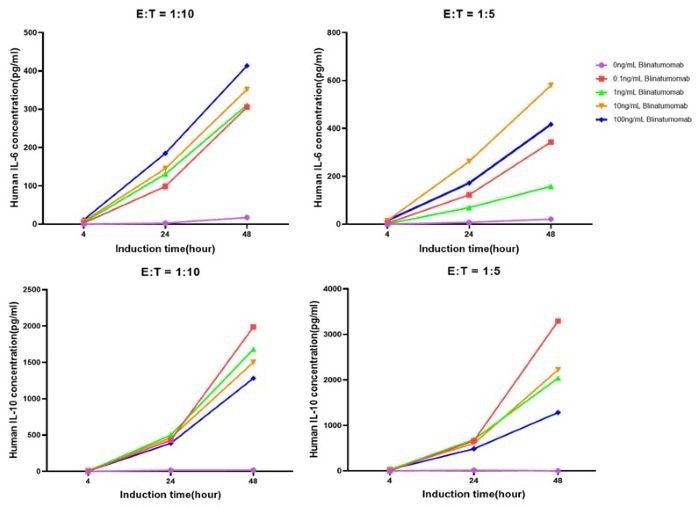
Figure 6. Elevation of IL-6 and IL-10 under Different Stimulation Conditions. Image Credit: ACROBiosystems
Product validation data
ClinMax™ Human IL-6 ELISA Kit (Cat. No. CRS-B001)
- Intra- and inter-assay precision: Figure 7 shows the CV of all measured quality control (QC) samples are less than 10%.
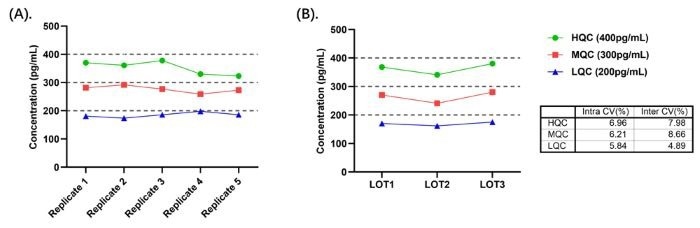
Figure 7. (A) Intra-Assay Precision Data for CRS-B001; (B) Inter-Assay Precision Data for CRS-B001. Image Credit: ACROBiosystems
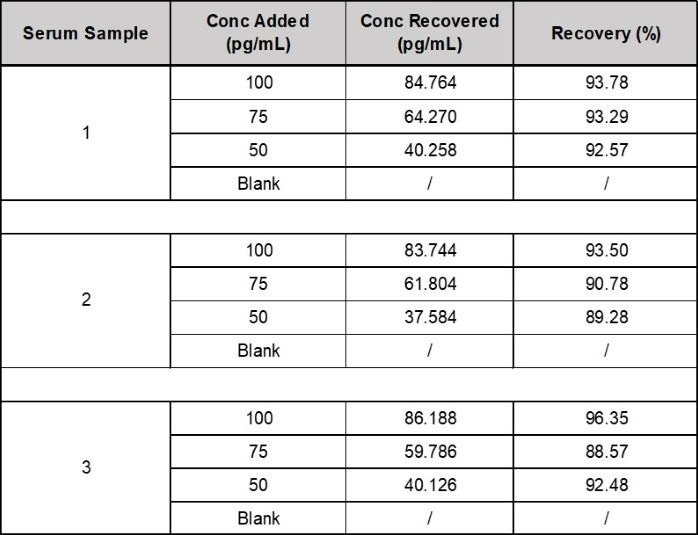
- Recovery: Three human serum samples were spiked with IL-6 before analysis. An average IL-6 recovery of 92.9% was collected from the serum samples. (see Table 1).
Table 1. Recovery of IL-6 in Spiked Samples. Source: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.