Thymic stromal lymphopoietin (TSLP) plays a key role in the immune response that contributes to the symptoms and clinical signs of asthma.
In response to various asthma triggers, TSLP is released from the airway epithelium, initiating an immune cascade that leads to airway narrowing, tightening, and increased inflammation—worsening asthma symptoms and potentially triggering asthma attacks.1,2
At the molecular level, TSLP signals travel through a heterodimeric receptor composed of the TSLP receptor (TSLPR) and the IL-7 receptor alpha chain (IL-7Rα). This receptor complex is expressed on a variety of immune and non-immune cells, including dendritic cells (DCs), mast cells, macrophages, basophils, T cells, epithelial cells, and neurons.
Subsequently, JAK1 and JAK2 primarily activate the signal transducers and activators of transcription STAT5A and STAT5B. This activation leads to the production of IL-4, IL-5, IL-9, and IL-13, along with other pro-inflammatory effects (Fig. 1).
During this activation process, TSLP exerts its biological function by binding to a high-affinity heteromeric receptor complex composed of the TSLP receptor chain and IL-7Rα. This interaction forms a ternary complex—TSLPR:TSLP:IL-7Rα—which initiates signaling in cells that co-express both TSLPR and IL-7Rα.
TSLP has been linked to a number of allergic diseases, including atopic dermatitis, bronchial asthma, and eosinophilic esophagitis.2 As a result, blocking the interaction between TSLP and its receptor complex has emerged as a promising strategy to inhibit TSLP function and prevent the overactive immune responses associated with allergic, eosinophilic, and other forms of airway inflammation in severe asthma.
Tezepelumab, developed by AstraZeneca in collaboration with Amgen, is a monoclonal antibody targeting TSLP that was approved for the treatment of severe asthma in 2021. This anti-TSLP monoclonal antibody drug blocks TSLP activity, acting at the initial end of the inflammatory cascade. Clinical data has shown that tezepelumab can improve symptoms considerably.4
The study discussed below introduces a number of methods suitable for the evaluation of anti-thymic stromal lymphopoietin biotherapeutics and biologics.
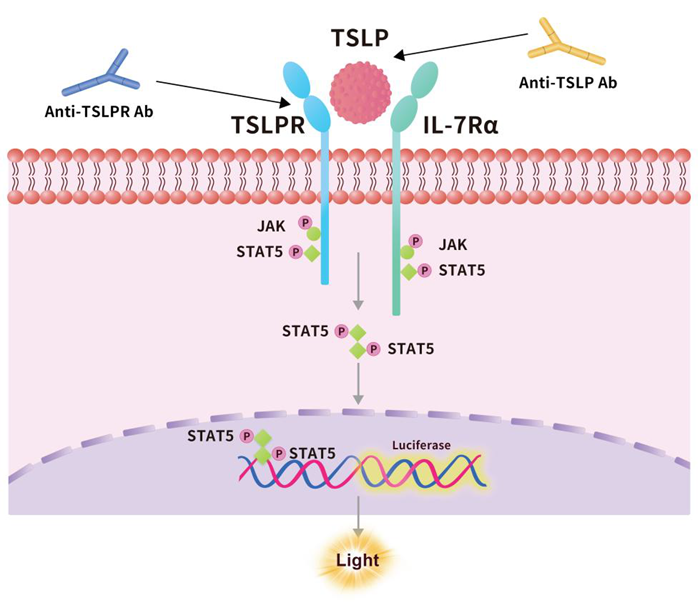
Figure 1. Mechanisms of thymic stromal lymphopoietin-induced signaling. Image Credit: Source: ACROBiosystems
TSLP-relevant receptors and molecular interactions
Lyophilized proteins were reconstituted in deionized water or PBS to prepare stock solutions, which were further diluted into working solutions as needed. Three different protein lots were used to assess lot-to-lot consistency.
In this study, a series of in vitro assays were conducted to characterize a TSLP inhibitor. One commercially available antibody was evaluated. The testing included two orthogonal assay types: one measuring TSLP binding to the TSLPR and IL-7R complex using BLI-based and ELISA methods, and the other assessing the antibody’s ability to inhibit downstream signaling.
Affinity validation via BLI assay
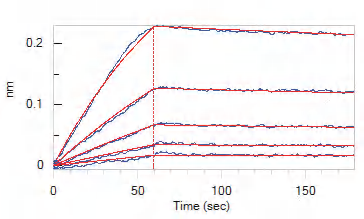
Figure 2. Loaded Human IL-7 RA&TSLP R Heterodimer Protein, Fc Tag&Fc Tag (Cat. No. ILR-H525x) on Protein A Biosensor, can bind Human TSLP Protein, His Tag, premium grade (Cat. No. TSP-H52Hb) with an affinity constant of 1.16 nM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: Source: ACROBiosystems
Inhibitor screening via neutralization assay (ELISA)
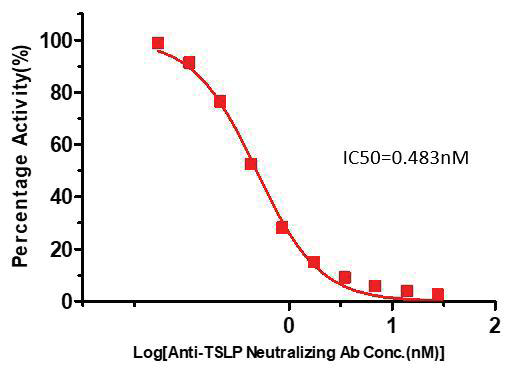
Figure 3. Inhibition of Biotinylated TSLP: IL-7R Alpha & TSLP R Binding by Anti-TSLP Neutralizing Antibody. Image Credit: Source: ACROBiosystems
Serial dilutions of anti-TSLP neutralizing antibody (Cat #EP129-C03) were prepared at a 1:1 serial dilution, from 8 µg/mL to 0.0156 µg/mL (55.317-0.108 nM). This dilution was added into IL-7Rα and TSLPR:TSLP-Biotin binding reactions before performing the assay with the defined protocol. The background was also subtracted from the data points before log transformation and curve fitting.
Inhibitor function validation via reporter assay
This reporter cell was incubated with serial dilutions of antibodies in the presence of human TSLP protein (Cat #TSP-H52Hb), resulting in a final concentration of 0.3 µg/mL. The EC50 of the anti-human TSLP neutralizing antibody was approximately 0.55 µg/mL in this instance.
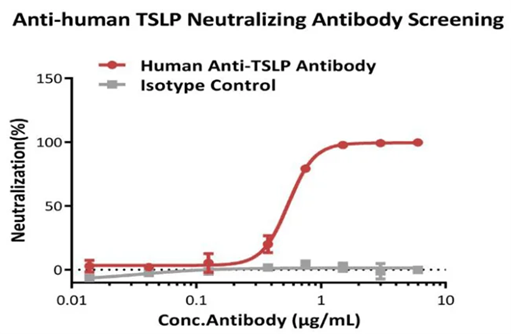
Figure 4. Inhibition of human TSLP protein-induced reporter activity by anti-human TSLP neutralizing antibody. Image Credit: Source: ACROBiosystems
Methods and materials
Table 1. BLI experimental Materials. Source: ACROBiosystems
| Materials |
Catalog. No. |
Vendor |
Human TSLP Protein,
His Tag, premium grade |
TSP-H52Hb |
ACROBiosystems |
Human IL-7 RA&TSLP R Heterodimer
Protein, Fc Tag&Fc Tag |
ILR-H525x |
ACROBiosystems |
| Protein A Biosensors |
18-5120 |
Sartorius |
96-well, flat bottom,
black polypropylene microplate |
655209 |
Greiner |
Bovine Serum Albumin
(IgG-Free, Protease-Free) |
001-000-162 |
Jackson |
| Tween-20 |
A600560-0500 |
Sangon Biotech |
| NaCl |
10017692 |
Sinopharm Chemical Reagent |
| KH2PO4 |
10019318 |
Sinopharm Chemical Reagent |
| Na2HPO4.12H2O |
10020392 |
Sinopharm Chemical Reagent |
| KCl |
10016392 |
Sinopharm Chemical Reagent |
| 10 mM Glycine-HCl, pH1.5 |
BR100354 |
Sinopharm Chemical Reagent |
BLI protocol
1. Baseline1
The biosensors were baselined in 1×PBS, pH 7.4, with 0.02 % Tween-20, 0.1 % BSA for a total of 60 seconds in Column 1 of the Greiner black microplate.
2. Loading ligand onto biosensors
Human IL-7 RA&TSLP R heterodimer protein, Fc Tag & Fc Tag (Cat #ILR-H525x), was diluted to 10 µg/mL with loading buffer (1×PBS, pH 7.4, with 0.02 % Tween-20, 0.1 % BSA) before being loaded onto the biosensors (2 nm value, loading for 100-500 seconds) in Column 2 of the Greiner black microplate.
3. Baseline2
The biosensors were baselined in sample dilution buffer (1×PBS, pH 7.4, with 0.02 % Tween-20, 0.1 % BSA) for a total of 180 seconds in Column 3 of the Greiner black microplate.
4. Analyte association
The analyte—Human TSLP Protein, His Tag, premium grade (Cat. No. TSP-H52Hb)—was diluted using Sample Dilution Buffer (1× PBS, pH 7.4, containing 0.02 % Tween-20 and 0.1 % BSA) to prepare seven concentrations: 25, 12.5, 6.25, 3.13, 1.56, and 0 nM.
These samples were added to wells A to G in column 4 of a Greiner black microplate.
The Human IL-7RA & TSLPR Heterodimer Protein, Fc Tag & Fc Tag (Cat. No. ILR-H525x), pre-loaded on biosensors, was then allowed to associate with the TSLP analyte at all seven concentrations simultaneously for 60 seconds.
5. Dissociation
Biosensors were dissociated in the sample dilution buffer (1×PBS, pH 7.4, with 0.02 % Tween-20, 0.1 % BSA) for a total of 120 seconds, with human TSLP protein, His Tag, premium grade (Cat #TSP-H52Hb) in Column 3 of the Greiner black microplate.
6. Regeneration
The biosensors ran a regeneration procedure, regenerating for 5 seconds in regeneration buffer (10 mM Glycine-HCl, pH 1.5) in Column 11 of the Greiner black microplate. This was followed by a 5-second neutralization in neutralization buffer (1×PBS, pH 7.4, with 0.02 % Tween-20, 0.1 % BSA) in Column 12 of the Greiner black microplate. This process was repeated three times.
ELISA protocol
1. Coating
The plate was coated with 0.1 µg/well (1 µg/ml, 100 µl/well) human IL-7 Rα and TSLPR at 4 °C overnight (or for 16 hours). The protein was diluted using the coating buffer.
2. Washing
Wells were washed three times, with 300 µl of washing buffer per well. The remaining solution was removed following washing via aspirating or decanting. The plate was inverted and sat on clean paper towels to ensure complete drying. This drying step is essential because the washing buffer must be removed completely.
3. Blocking
The wells were blocked with 300 µl of blocking buffer per well at 37 °C for a total of 1.5 hours.
4. Second washing
A further washing step was completed, as before.
5. Sample addition
- Biotinylated human TSLP stock solution (50 µg/mL) was diluted to 0.0035 µg/mL with dilution buffer in order to create a working solution of biotinylated human TSLP.
- Anti-TSLP neutralizing antibody stock solution was diluted with dilution buffer from 0.016-8.0 µg/mL before being mixed with an identical volume of biotinylated human TSLP working solution. For example, 110 µL biotinylated human TSLP working solution + 110 µL diluted anti-TSLP neutralizing antibody.
- The samples were diluted in series as appropriate before being mixed with an identical volume of biotinylated human TSLP working solution. For example, 110 µL biotinylated human TSLP working solution + 110 µL diluted samples.
- A total of 100 µL of mixer was added to the wells before sealing the plate using microplate sealing film. This was then incubated at 37 °C for 1 hour.
6. Third washing
A third washing step was completed, as before.
7. Adding streptavidin-HRP
- Streptavidin-HRP stock solution (50 µg/mL) was diluted to 0.1 µ g/mL with dilution buffer in order to create a working solution of streptavidin-HRP.
- For every well, 100 µL Streptavidin-HRP working solution was added before sealing the plate with microplate sealing film and incubating at 37 °C for 1 hour. Light was also avoided during this step.
8. Fourth washing
A fourth washing step was completed, as before.
9. Adding substrate
A total of 100 µl of substrate solution was added into each well before being incubated at 37 °C for 20 minutes. Again, light was avoided.
10. Termination
A total of 50 µl of sulfuric acid was added to each well at 1 mol/L in order to stop the reaction.
11. Read and calculate
Absorbance was read at 450 nm using a UV/Vis microplate spectrophotometer.
Table 2. Neutralization assay-ELISA Experimental Materials. Source: ACROBiosystems
| Buffer Name |
Catalog. No. |
|
Loading
Buffer |
1×PBS, pH7.4, with 0.02% Tween-20, 0.1%BSA (10 mM Na2HPO4.12H2O, 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, 0.02 % Tween-20, 0.1 %BSA, pH 7.4) |
Baseline1: Baseline the Biosensors in Loading buffer for 60 s. |
| Loading: Dilute Human IL-7 RA&TSLP R Heterodimer Protein, Fc Tag&Fc Tag (Cat. No. ILR-H525x) to 10 μg/mL with Loading Buffer, and Loading about 2.0 nm onto the Biosensors. |
Sample
dilution
Buffer |
1×PBS, pH7.4, with 0.02 %
Tween-20, 0.1 %BSA (10 mM Na2HPO4.12H2O, 2 mM
KH2PO4, 137 mM NaCl,
2.7 mM KCl, 0.02 % Tween-20, 0.1 %BSA, pH 7.4) |
Baselin2: Baseline the Biosensors in Sample Dilution Buffer for 180 s. |
Association: Dilute the analyte Human TSLP Protein, His Tag,
premium grade (Cat. No. TSP-H52Hb) with the Sample Dilution Buffer from 25 to 1.56 nM, and association for 60 s. |
Dissociation: The Biosensors dissociate in Sample Dilution
Buffer for 120 s. |
Regeneration
Buffer |
10 mM Glycine-HCl, pH1.5 |
Regeneration: 3 times Regeneration in 10 mM Glycine-HCl, pH1.5 for 5 s. |
Neutralization
Buffer |
1×PBS, pH7.4, with 0.02%
Tween-20, 0.1 %BSA (10 mM Na2HPO4.12H2O, 2 mM KH2PO4, 137 mM NaCl,
2.7 mM KCl, 0.02% Tween-20, 0.1 %BSA, pH 7.4) |
Neutralization: Neutralization for 5s in neutralization buffer immediately after each regeneration. |
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
References and further reading
- Corren, J. and Ziegler, S.F. (2019). TSLP: from allergy to cancer. Nature Immunology, (online) 20(12), pp.1603–1609. https://doi.org/10.1038/s41590-019-0524-9.
- Ebina-Shibuya, R. and Leonard, W.J. (2023). Role of thymic stromal lymphopoietin in allergy and beyond. Nature Reviews Immunology, (online) 23(1), pp.24–37. https://doi.org/10.1038/s41577-022-00735-y.
- Reynold Panettieri Jr, et al. (2024). Tezepelumab for Severe Asthma: One Drug Targeting Multiple Disease Pathways and Patient Types. Journal of asthma and allergy, Volume 17, pp.219–236. https://doi.org/10.2147/jaa.s342391.
- Shaban Abdelgalil, M., et al. (2022). Safety and efficacy of tezepelumab vs. placebo in adult patients with severe uncontrolled asthma: a systematic review and meta-analysis. Scientific Reports, 12(1). https://doi.org/10.1038/s41598-022-24763-9.
- Verstraete, K., et al. (2017). Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nature Communications, 8(1). https://doi.org/10.1038/ncomms14937.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.