CAR-T cell therapy has brought hope of a cure to a large number of cancer patients in recent years. Despite its outstanding effectiveness, the toxicity known as cytokine release syndrome (CRS) remains a serious problem.
Once CAR-T has been infused into the human body, this activates T lymphocytes, which rapidly proliferate, prompting an excessive release of cytokines such as TNF-α, IFN-γ, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, and IL-12.
This cytokine release can result in a number of outcomes, for example, dyspnea, hypotension, myalgia, fever, blood coagulation disorders, and end-organ dysfunction. These outcomes have the potential to cause permanent damage to organs and tissues, even resulting in death.
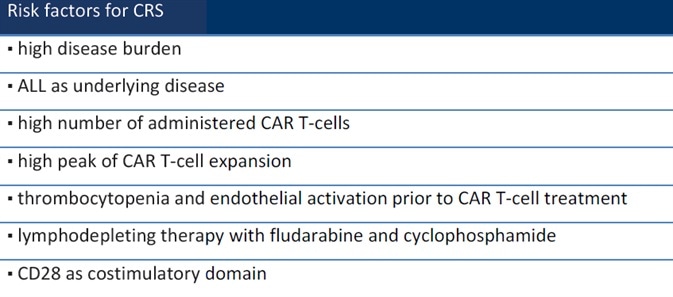
While CRS is an acute systemic inflammatory syndrome associated with CAR-T cell therapy,1 a number of factors can impact a patient’s chances of having CRS. These include treatment procedure, disease state, and drugs2 (Table 1).
Table 1. Risk factors for CRS. Source: ACROBiosystems
Clinical identification of CRS onset
CRS patients tend to exhibit increased levels of ferritin and serum CRP, meaning that these may be utilized as biomarkers when determining the occurrence of CRS.
Each of these markers, including ferritin, LDH, CRP, AST, ALT, and creatinine, will only appear following clinical manifestations of CRS. It is not possible to detect the onset of CRS via these markers.
The majority of hospitals are unable to monitor cytokine levels in real-time; they are only able to observe clinical manifestations. The timescales around CRS occurring are impacted by a number of factors, for example, the patient’s disease state or type of CAR-T therapy.
CRS peaks on the 3rd and 7th day after administration of Kymriah, which includes the costimulatory domain of 4-1BB (CD137).
CRS occurs on the second day of administration of Yescarta or Tecartus, which includes the costimulatory domain of CD28. Some instances of delayed CRS were reported after three weeks of CAR-T administration, however.2
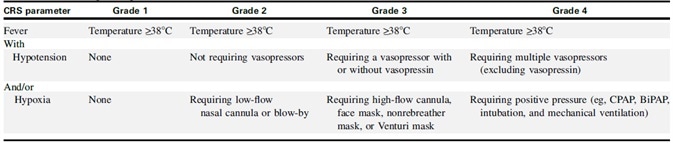
The American Society for Transplantation and Cell Therapy (ASTCT) established a CRS consensus grading guideline in 2019 (Table 2). This guideline states that fever (≥38 °C) is a requirement. Conditions become more severe, either with or without hypotension and/or hypoxia.
ASTCT grading
According to a number of studies, employing the ASTCT grading system provides an accurate, objective, and repeatable rapid clinical assessment of CRS clinically severity.2,3 A summary of the grading guideline is provided below.
Table 2. ASTCT CRS Consensus Grading. Source: ACROBiosystems
Grade 1
Grade 1 includes CRS patients displaying fever and some non-specific signs but without hypotension or hypoxia. Treatment strategies at this grade involve intravenous infusion of antipyretic drugs.
Grade 2
Grade 2 CRS is defined as fever (≥38.0 °C) with hypotension that does not require vasopressors and/or hypoxia that requires the use of oxygen delivered by blow-by or low-flow nasal cannula (≤6 L/minute).
It should be noted, however, that CRS patients are at risk of edema as a result of capillary leakage or vasodilatation and that fluid infusion should be closely monitored in order to avoid this.
Tocilizumab inhibits IL-6, and this can be used for Grade 2 patients. Early use of tocilizumab has been shown to reduce the prevalence of severe CRS and organ failure while maintaining CAR-T therapy efficacy.
Grade 3
Grade 3 CRS patients are those that exhibit low blood pressure, fever, and no response to fluid infusion. This grade also includes patients requiring high flow oxygen (≥6 L/min).
These patients should be considered for entry into an intensive care unit (ICU) for management and care, with Belizumab and glucocorticoids (dexamethasone, etc.) utilized for treatment.
It is recommended to use a vasopressor (such as norepinephrine) and/or high-flow nasal cannula (>6 L/minute) in order to reduce the risk of further deterioration and visceral damage. High-grade CRS patients are also prone to cardiac dysfunction, so the cardiac function should be closely monitored.
Grade 4
Grade 4 CRS patients are defined as having a fever (≥38.0 °C) with hypotension, necessitating the use of multiple vasopressors (excluding vasopressin) and/or hypoxia that requires positive pressure; for example, mechanical ventilation, bilevel positive airway pressure, CPAP, intubation. These symptoms should not be attributable to any other cause.
Methylprednisolone is recommended to use at a dose of 1 g/day in addition to any treatment for Grade 3 CRS. According to studies, high-grade CRS is reversible through appropriate early intervention.
Roche's drug tocilizumab (trade name: Actemra) was approved by the FDA in 2017. It has been designed for the CRS caused by CAR-T therapy, mitigating this by blocking the inflammatory cytokine interleukin-6 (IL -6).
Clinical guidance for tocilizumab4
A range of clinical guidance has been provided for tocilizumab. Tocilizumab should be considered in the early stage of CRS for elderly patients or those with severe complications. The drug can be administered for adults with Grade 2 CRS, as well as children with Grade 3 CRS. It can also be administered to pediatric patients developing Grade 2 CRS but intolerant to fever.
If adults and children are administered one dose of tocilizumab and conditions fail to improve, the second dose should be administered together with glucocorticoids. Should there continue to be no improvement, Siltuximab, Anakinra, and a high dose of methylprednisolone should be considered.
CRS treatment
Researchers continue to work hard on the mitigation and effective treatment of CRS. Prominent journal Nature published two articles on a new method of controlling CAR-T toxicity on the same day in 2018, outlining research that confirmed that inhibiting IL-1 can reduce macrophage-induced CRS.
An article was published in Science Translational Medicine in 2019 by Michel Sadelain, director of the Cell Engineering Center at Sloan Kettering Cancer Center. Within the article, he identified a functional switch in CAR-T cells that were found to help avoid CRS in mouse models.5
A research team from the Chinese Academy of Medical Sciences published an article in Science Immunology in January 2020, outlining the process of pyrolysis caused by CAR-T therapies, alongside the mechanism of CRS. Their clear microscope images confirmed that the whole process of CRS was triggered by CAR-T cell therapy.6
Significant breakthroughs can be anticipated soon, thanks to continuous clinical research into CRS and CAR-T therapies.
ACROBiosystems has developed a range of CAR-T target proteins, including EGFR, CD19, BCMA, CD22 MSLN. Each of these proteins is available in different forms such as non-labeled, fluorescent-labeled, and biotin-labeled. Flow cytometry protocols for CAR expression detection are also provided.
ACRO FITC-labeled CD19 protein (Cat. No. CD9-HF2H2) is highly sensitive and specific in anti-CD19 CAR detection assays. Another antibody is available (Cat. No. FM3-FY45), which targets the antigen-binding epitope of FMC63 scFv, therefore providing an additional means of Anti-CD19 (FMC63) CAR detection.
Furthermore, ACRO FITC-labeled BCMA protein (Cat. No. BCA-HF254) offers good sensitivity, is well-recognized, and has been used in a wide range of different assays.
Product list
Table 3. CD19. Source: ACROBiosystems
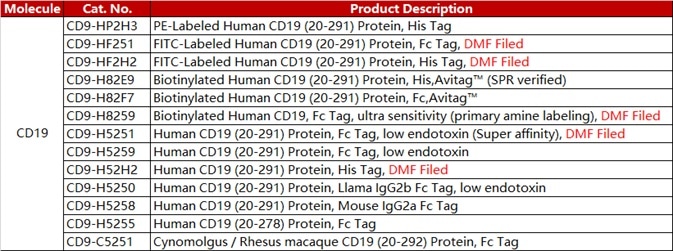
Table 4. BCMA. Source: ACROBiosystems
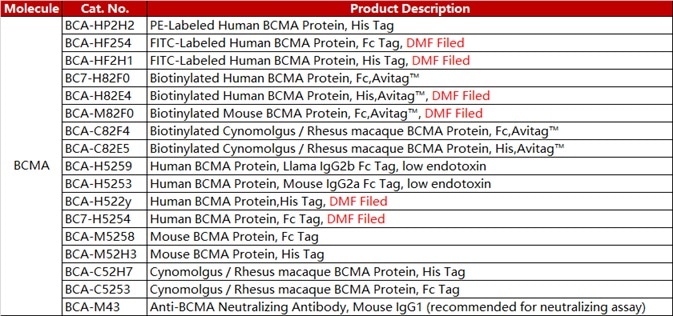
Table 5. Anti-FMC63 mAb. Source: ACROBiosystems
Case study
Within this example study, 1e6 of anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was utilized as a negative control.
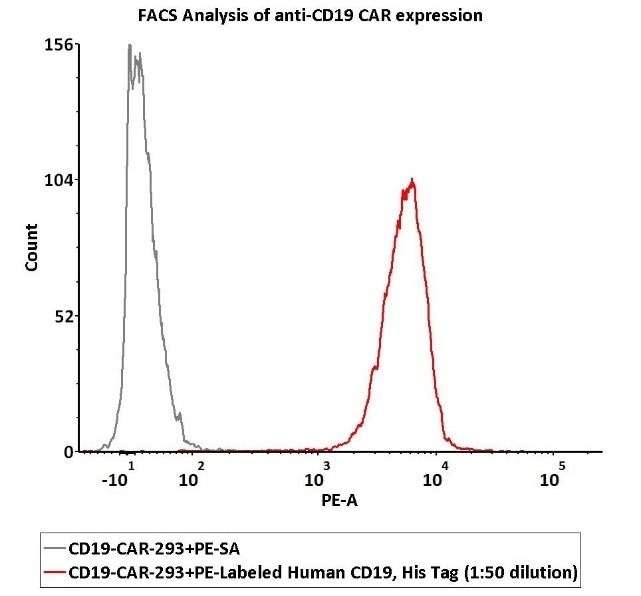
Image Credit: ACROBiosystems
2e5 of Anti-CD19 CAR-293 cells were stained using 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control respectively. FITC signal was employed to evaluate binding activity.
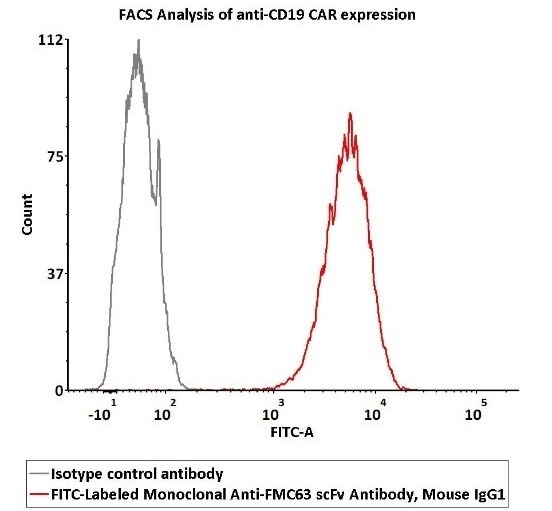
Image Credit: ACROBiosystems
1e6 of the Anti-BCMA CAR-293 cells were stained using 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No. BCA-HP2H2) and negative control protein respectively. Here, PE signal was employed in order to evaluate binding activity.
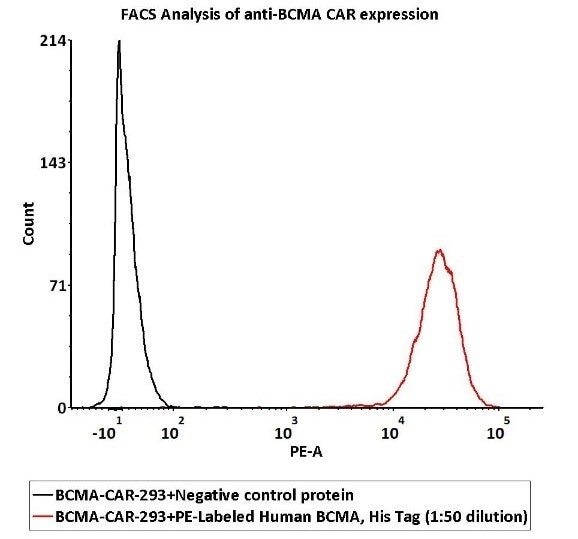
Image Credit: ACROBiosystems
References and Further Reading
- https://www.cn-healthcare.com/articlewm/20190306/content-1047067.html.
- Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34-48.
- Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146(5):940-948.
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511.
- Mestermann, Katrin, et al. "The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells." Science translational medicine 11.499 (2019).
- Liu, Yuying, et al. "Gasdermin E–mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome." Science immunology 5.43 (2020).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.