For the past 20 years, the quick development of biological therapeutics, like antibodies, CAR-T cell therapy, antibody-drug conjugates, etc., have played a significant role in treating several significant diseases.
But as an exogenous entity, such drugs can produce anti-drug antibodies after being injected into the body. This might decrease the efficacy of drugs and, in several cases, might be dangerous. Thus, pharmacokinetic (PK) analysis and Immunogenicity (ADA) research are crucial in the development of biopharmaceuticals.
Anti-idiotypic antibodies are the main tool reagents for PK/ADA assay. Specifying the development plan of an anti-idiotypic antibody is significant in achieving a precise and efficient bioassay technique to guarantee that a project is completed on time.
This article outlines the development plans of anti-idiotypic antibodies for various kinds of biological drugs to help users successfully develop their bioanalytical techniques.
Development strategy of anti-idiotypic antibodies for monoclonal antibody drugs
With several years of development, monoclonal antibodies (mAb) have slowly changed from scientific research tools (reagents) to biological drugs that offer a new therapeutic modality for a range of diseases. Coming up with anti-idiotypic antibodies is significant in studying a mAbs safety and effectiveness.
Table 1. Source: ACROBiosystems
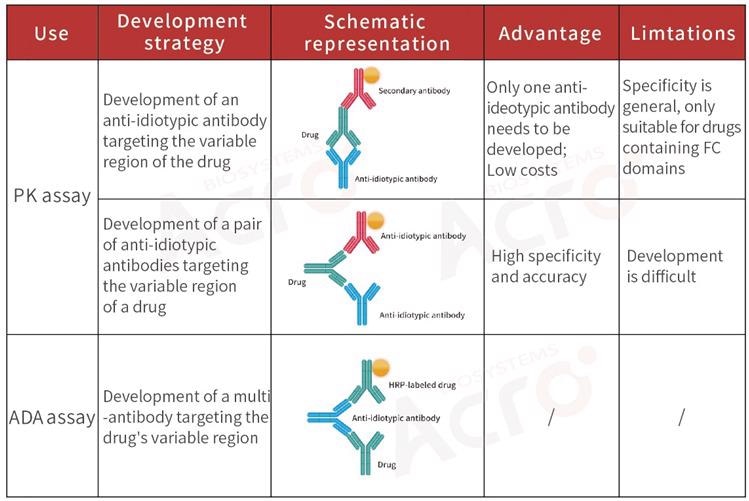
Development strategy of anti-idiotypic antibody for bispecific antibodies
Bispecific antibody: An altered antibody consisting of two specific antigen-binding sites. The pharmacokinetic analysis of the drug is highly complicated due to its two diverse variable regions.
Table 2. Source: ACROBiosystems
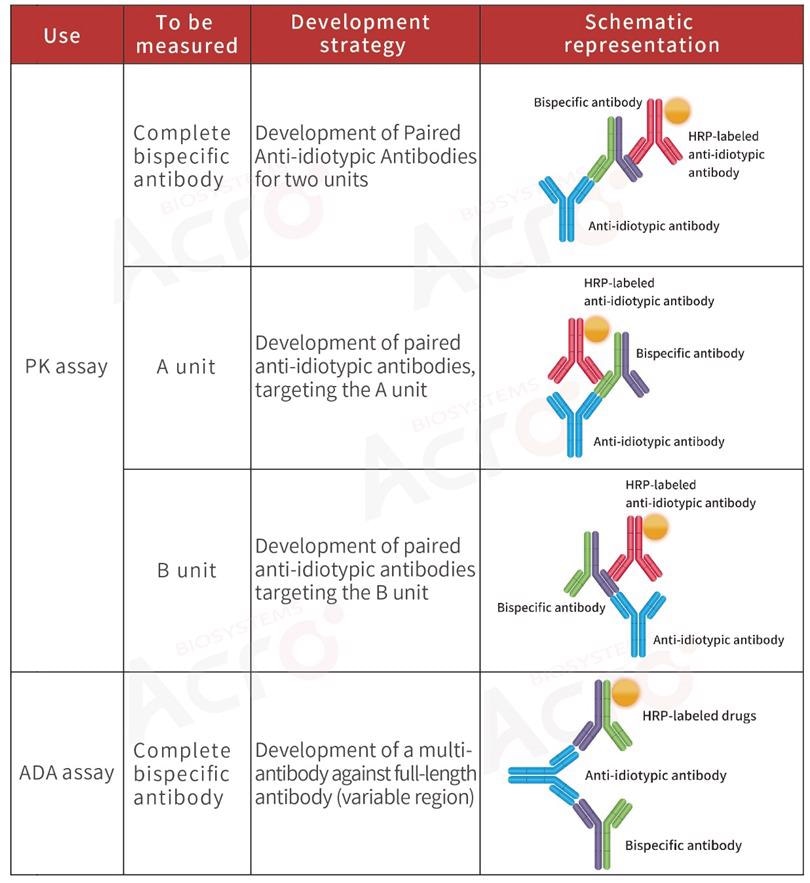
To better assess the safety and efficacy of antibody drugs, it is usually suggested that scientists develop anti-idiotypic antibodies for the full bispecific antibody while the PK analysis is being executed.
Development strategy of anti-idiotypic antibody for ADC drugs
Antibody-drug conjugates (ADCs) are innovative biopharmaceutical products in which a monoclonal antibody has been associated with a small molecule drug with a stable linker.
As a result of the ADC's structural specificity and complexity, the assessment of PK for ADCs needs a special and highly complicated bioassay protocol compared to traditional monoclonal antibodies and small molecular drugs.
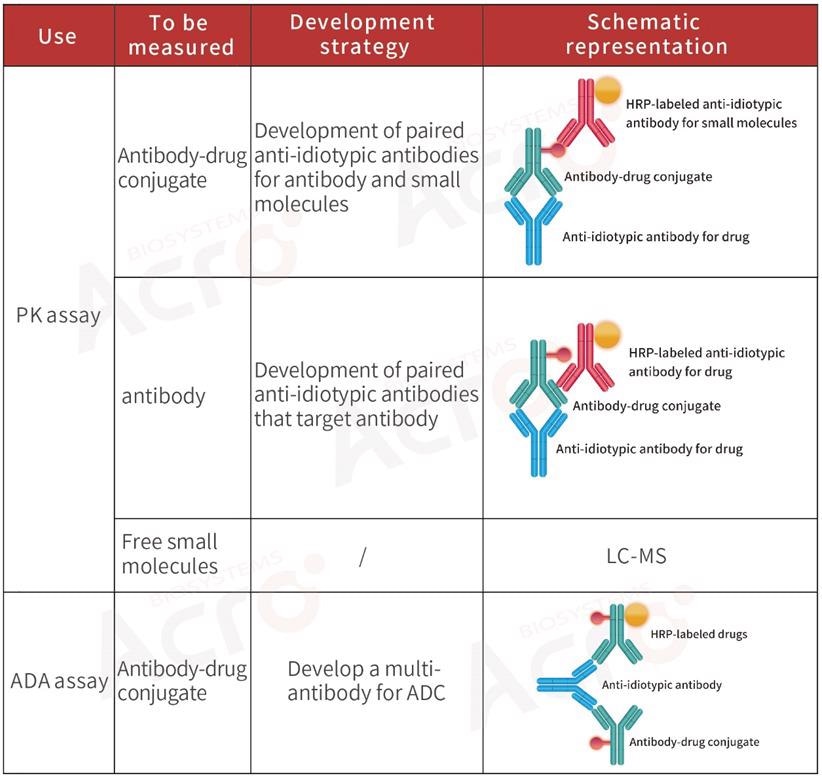
Normally, PK analysis of ADCs detects three main components: total ADC drugs, total antibodies, and free small molecular cytotoxic drugs (with or without linker). Scientists normally utilize ligand-binding assay for ADCs and total antibodies to evaluate the effectiveness and targeted toxicity of ADCs.
For the safety and stability evaluation of ADCs, that is, the toxicity of such drugs to non-tumor cells in vivo, it is essential to measure the free small molecular toxicity.
Table 3. Source: ACROBiosystems
Development strategy of anti-idiotypic antibody for CAR-T drugs
Chimeric antigen receptor T (CAR-T) cell immunotherapy. CAR-T cells are the antigen-binding parts of antibodies that can identify a specific tumor antigen with the intracellular part of the CD3-ζ chain or FcεRIγ in vitro linked to a chimeric protein. CAR was expressed in T cells of patients by gene transduction.
For CAR-T, flow cytometry (FCM) and real-time fluorescence quantitative polymerase chain reaction (qPCR) were utilized for PK analysis to identify the foreign gene copy and various CAR + cells, respectively. Cell-based assays are utilized to screen anti-idiotypic antibodies against genetically engineered scFv antibodies used for PK analysis of CAR-T.
Table 4. Source: ACROBiosystems

Development strategy of anti-idiotypic antibody for biosimilar
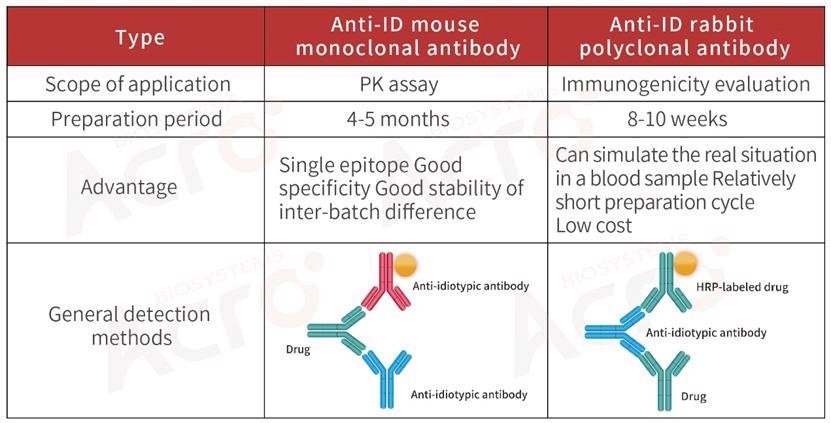
For the PK or ADA assays of biosimilar drugs, anti-idiotypic antibodies can be developed as per the variable region of the drug, or the original ADA product can be purchased.
ACROBiosystems has come up with a series of high-affinity, high-specific anti-idiotypic antibodies, and PK blood concentration quantitative detection kits used for immunogenicity analysis and pharmacokinetic studies to aid these studies.
ACROBiosystems will come up with the equivalent protocol as per the different application scenarios for every anti-idiotypic antibody. The hope is to expedite the drug development process to the highest extent. The products developed cover rituximab, adalimumab, cetuximab, bevacizumab, trastuzumab, and several other popular antibody drugs.
ACROBiosystems, as a worldwide brand of protein technologies, products, and services focused on biologics, is committed to offering targeted antigens, other key reagents, and related services needed in the development process of targeted therapeutic drugs.
Table 5. Source: ACROBiosystems
For the different needs of customers to be fulfilled, the Company can also offer one-stop service from antigen preparation to polyclonal anti-idiotypic antibody, pharmacokinetics, monoclonal anti-idiotypic antibodies, and immunogenicity test kit development.

Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.