October 29, World Psoriasis Day, was established to raise global awareness and promote an accurate understanding of psoriasis (PsO). The chronic, recurrent autoimmune disease is commonly associated with comorbidities that impact both the skin and systemic organs.

Image Credit: ACROBiosystems
PsO affects over 125 million individuals around the world. Its complex immune pathogenesis and significant disease burden make the need for safer, more effective targeted treatments especially important.
PsO is a systemic inflammatory disease that presents not only as scaly skin lesions, erythema, and itching but also affects the joints, cardiovascular system, and metabolism. Plaque psoriasis is the most common clinical type, accounting for more than 80 % of all cases, with patients commonly experiencing intense itching, burning, and pain.
Without proper management, around 30 % of patients may further develop psoriatic arthritis (PsA), which can lead to irreversible joint damage. Addressing this serious challenge requires advanced therapeutic approaches targeting the underlying immune mechanisms to precisely interrupt the inflammatory cascade.
ACROBiosystems has built an extensive product portfolio for PsO studies, including high-activity recombinant proteins, stable cell lines, and inhibitor screening kits.
These research tools support every stage of therapeutic development, including target discovery and validation, candidate drug screening and development, and CMC production and quality control. This portfolio accelerates the efficient translation of PsO novel treatments from foundational research to clinical implementation.

Image Credit: ACROBiosystems
At the center of an immune storm: From genetic triggers to cytokine crossfire
PsO represents a chronic, immune-driven storm fueled by the interactions between the innate and adaptive immune systems. At its core is the IL-23/IL-17 axis, a critical pathway that promotes inflammation and tissue damage. In individuals who are genetically predisposed to the disease, everyday triggers such as skin injury or infection can cause this immune cascade.
Damaged skin cells release danger signals (DAMPs), alerting dendritic cells (DCs) and other antigen-presenting cells. After activation, these DCs produce IL-23, which drives TH17 and γδ T cells to secrete potent inflammatory mediators, IL-17A, IL-17F, and IL-22. Among these, IL-17 functions as the “final enforcer,” directly causing excessive growth and abnormal differentiation in keratinocytes.
This process results in a feedback loop: keratinocytes produce more chemokines and antimicrobial peptides, attracting immune cells that further intensify inflammation. In combination with TNF-α, IFN-γ, and other cytokines, this network forms a tightly woven inflammatory circuit responsible for the hallmark psoriasis plaques – and often spills beyond the skin to induce systemic inflammation.
PsO treatment: From TNF-α inhibitors to precision biologics targeting IL-23 and IL-17
The PsO therapeutic landscape has moved from an era of traditional systemic therapeutics and TNF-α inhibitors to a realm of precision medicine, dominated by emerging targets including IL-23 and IL-17. This change has diversified targets, accelerated drug iteration, and increased homegrown therapies, fundamentally changing market dynamics.
In the early days of biologics, TNF-α inhibitors, including AbbVie’s adalimumab, Johnson & Johnson’s infliximab and golimumab, and Amgen/Pfizer’s etanercept, were market leaders due to their broad anti-inflammatory impacts. But patent expiry and a rise in biosimilars have caused their steady market decline.
A breakthrough came with Johnson & Johnson’s ustekinumab, the world’s first biologic to target the IL-12/IL-23 p40 subunit. This success confirmed the IL-23 pathway’s central role in PsO pathogenesis, paving the way for more accurate treatments.
As researchers gain a deeper understanding of PsO’s mechanisms, the focus has shifted toward IL-23 inhibitors targeting upstream cytokines and IL-17 inhibitors acting directly on downstream inflammation. These therapies have become the primary treatment options on the market today.
IL-23 inhibitors, including Johnson & Johnson’s guselkumab (projected 2024 global sales of $3.6 billion, +16.6 % YoY), AbbVie’s risankizumab, and Eli Lilly’s mirikizumab, selectively target the p19 subunit of IL-23.
These agents deliver excellent efficacy and long-lasting remission, setting a high standard for novel therapies.
In contrast, IL-17 inhibitors, such as Novartis’ secukinumab (2024 sales exceeding $6.2 billion), Eli Lilly’s ixekizumab (more than $3.6 billion), and UCB’s bimekizumab, neutralize IL-17A, resulting in rapid onset of action and high skin clearance rates. These therapeutics have established a strong market presence with high clinical response rates, creating a significant barrier for competitors.
Approved originator biologics for PsO. Source: Pharmacodia
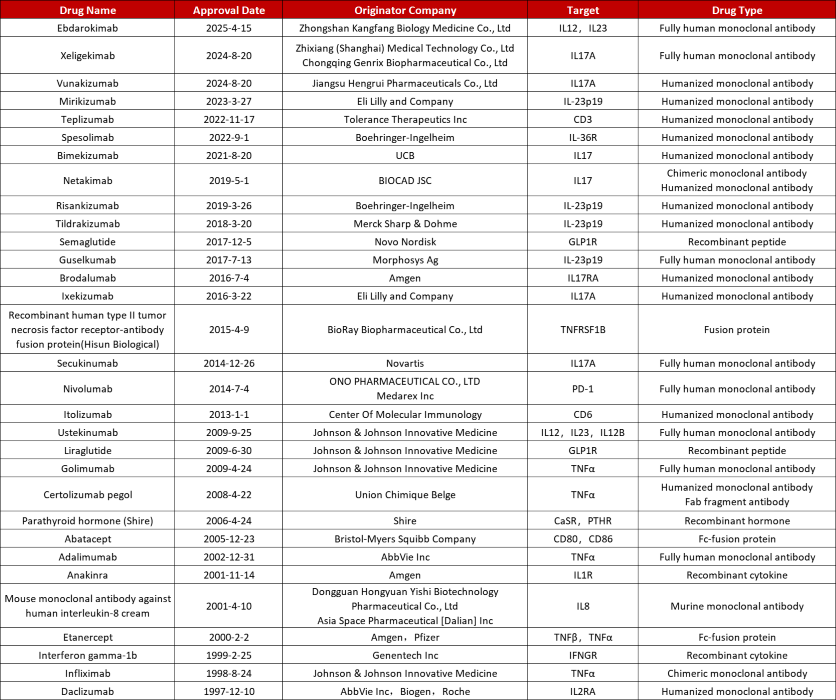
While international companies have traditionally dominated the market, emerging targeted therapies are accelerating breakthroughs while diversifying the therapeutic landscape.
A particularly interesting example is Boehringer Ingelheim’s pemafostat, the first IL-36R inhibitor to receive global approval for generalized pustular psoriasis (GPP).
This innovative therapy fills a crucial gap in the targeted treatment of this rare and severe condition, highlighting the industry's growing trend toward precision treatments tailored to specific patient populations.
Many biopharmaceutical companies have made notable advancements in novel therapeutic development over the past few years, with several products achieving global or regional market approvals, demonstrating increasingly advanced research and development capabilities.
For example, in August 2024, Zhixiang’s secukinumab and Hengrui’s furenikizumab, targeting IL-17A, both received market approval, allowing them to enter the mainstream market to compete in this high-demand area.
Approved in April 2025, Kangfang Bio’s irukizumab simultaneously inhibits IL-12 and IL-23’s p40 subunit, similar to the mechanism of the early global blockbuster ustekinumab. Its dual-target mechanism offers strong clinical potential and promising market opportunities.
ACROBiosystems pioneers PsO therapeutics with innovative solutions
ACROBiosystems supports PsO research with their comprehensive range of research tools, which include high-activity recombinant proteins, stable cell lines, and inhibitor screening kits.
These solutions cover the complete drug development process – including target discovery and validation, candidate drug screening and development, CMC production, and quality control – accelerating the efficient translation of cutting-edge PsO treatments from basic research to clinical application.

Image Credit: ACROBiosystems
Hot PsO target recommendations
Source: ACROBiosystems
| |
|
|
|
| PD-1 |
IL23A & IL12B |
TNF-alpha |
IL-6 |
| IL-17A |
IL-23R |
IL-2 R beta & IL-2 R alpha &
IL-2 R gamma |
IL-12B |
| IL-1 Rrp2 |
IL-1 RI |
IL-22 R alpha 1 |
TNF-beta |
| L-17F |
CD40 |
IL-10 R alpha |
CD3E & CD3G |
| CD3 gamma |
CD4 |
CCR6 |
EGFR |
| ICAM1 |
IL-12R |
IL-17RA |
IL-18 |
| IL-12A |
IL-2 R beta |
VEGF |
BTLA |
| Complement C3 |
|
|
|
References and further reading:
- Sieminska, I., Pieniawska, M. and Grzywa, T.M. (2024). The Immunology of Psoriasis-Current Concepts in Pathogenesis. Clinical Reviews in Allergy & Immunology, (online) 66. DOI: 10.1007/s12016-024-08991-7. https://link.springer.com/article/10.1007/s12016-024-08991-7.
- Jiang, Y., et al. (2023). Biologic and Small-Molecule Therapies for Moderate-to-Severe Psoriasis: Focus on Psoriasis Comorbidities. BioDrugs, (online) 37(1), pp.35–55. DOI: 10.1007/s40259-022-00569-z. https://link.springer.com/article/10.1007/s40259-022-00569-z.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empowers scientists and engineers dedicated to innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.