The innovative drug market has expanded significantly in recent years, with continued growth projected through to 2031.
Current research and development (R&D) has demonstrated the market's unprecedented potential and vitality, fuelled by an array of policy incentives and capital market support.
This has led to a significant number of pharmaceutical companies actively investing in the innovative drug area, engaging in fierce competition for market share.
As patient populations and market demand continue to rise, companies need distinctive R&D pipelines and precise therapeutic targeting to stand out in this competitive field.
Image Credit: Market Research Intellect
Multi-pass transmembrane proteins
Transmembrane proteins (TPs), also referred to as ‘integral membrane proteins’, make up between 70 % and 80 % of the total TPs. They are a key drug target in current R&D, accounting for more than 60 % of all targets. These proteins account for over 90 % of antibody drug targets.
Among them, structurally complex multi-pass transmembrane proteins (such as GPCRs, ion channels, and transporters) are key to material transport and signal transduction.
They are closely linked to a range of conditions, including neurodegenerative, autoimmune, and developmental disorders, as well as cancers.
A number of active research projects are currently focused on multi-pass TPs, due to their excellent potential to enable future targeted therapeutic options.
Multi-pass TPs as prevalent targets for cutting-edge drug R&D
CD20: A popular target in the bispecific antibody field
CD20 is a four-pass TP primarily expressed on the surface of B lymphocytes. CD20 is an important marker for B-cell lymphomas, making it a key target for a range of anti-lymphoma drugs.
However, CD20's application is not limited to monoclonal antibody therapies. Combining CD20 with CD3 targets has enabled the development of bispecific antibodies (bsAbs), which are able to recruit T cells to the vicinity of B cells. This effectively allows T cells to kill B cells.
CD3/CD20 bispecific antibodies are a widely studied target combination, offering a promising treatment option for multiple myeloma (MM), refractory or relapsed non-Hodgkin lymphoma (NHL), and other cancers where drug resistance remains challenging.
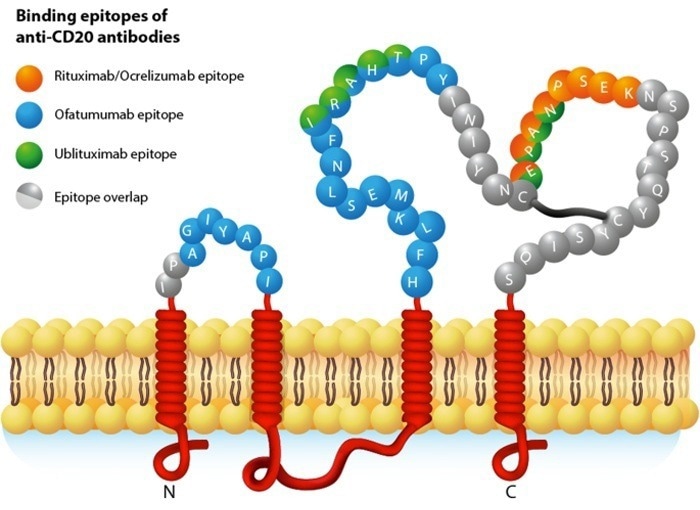
Diagram of CD20 Structure. Image Credit: ACROBiosystems
Claudin 18.2: A new hope for gastric cancer treatment
The four-pass TP Claudin 18.2 belongs to the Claudins family. While Claudin 18.1 is primarily expressed in normal lung tissue, Claudin 18.2 is specifically expressed in differentiated gastric mucosal epithelial cells.
Claudin 18.2 is not expressed in gastric stem cell regions, a key difference that improves the treatment potential of therapeutic antibodies targeting Claudin 18.2 while reducing the toxicity of gastric cancer therapies.
On 18 October 2024, the United States Food and Drug Administration (FDA) approved Zolbetuximab, the first monoclonal antibody targeting Claudin 18.2.
It is intended for use alongside chemotherapy to treat Claudin 18.2-positive advanced, unresectable, or recurrent gastric cancer and gastroesophageal junction adenocarcinoma. This approval is the first and currently only targeted treatment option available for Claudin 18.2-positive gastric cancer patients.
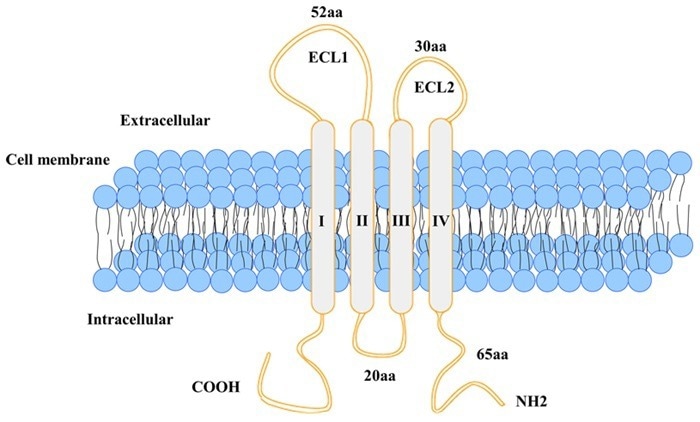
Diagram of Claudin 18.2 Structure. Image Credit: ACROBiosystems
GPRC5D: An ideal target for multiple myeloma
The seven-pass TP, GPRC5D, is a key G protein-coupled receptor (GPCR) that is highly expressed in malignant plasma cells but limited to hair follicle regions in normal tissues.
This gives GPRC5D a high degree of specificity and makes it an ideal target for designing immunotherapy strategies for multiple myeloma. A number of immunotherapeutic approaches targeting GPRC5D are currently being developed, including CAR-T therapies and T-cell redirecting bsAbs.
GPRC5D is an emerging drug target that may lead to breakthroughs in treating multiple myeloma.
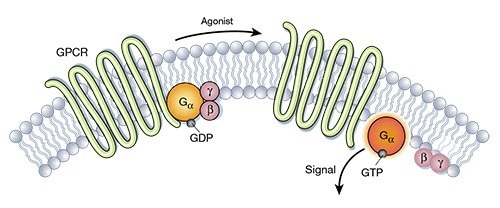
Diagram of GPCRs Structure. Image Credit: ACROBiosystems
Claudin 6: A potential target for solid tumors
Claudin 6 is a member of the Claudins family that is highly and specifically expressed in a range of solid tumor tissues, including testicular, ovarian, endometrial, liver, gastric, and non-small cell lung cancer.
Its expression negatively correlates with prognosis but is almost absent in normal adult tissues.
Its strict silence in healthy adult tissues and its abnormal activation and high-level expression in a range of solid tumors with high medical needs have seen Claudin 6 become a focal point in multiple drug research fields. It is also considered one of the most promising anticancer therapy targets in the Claudin family, after Claudin 18.2.
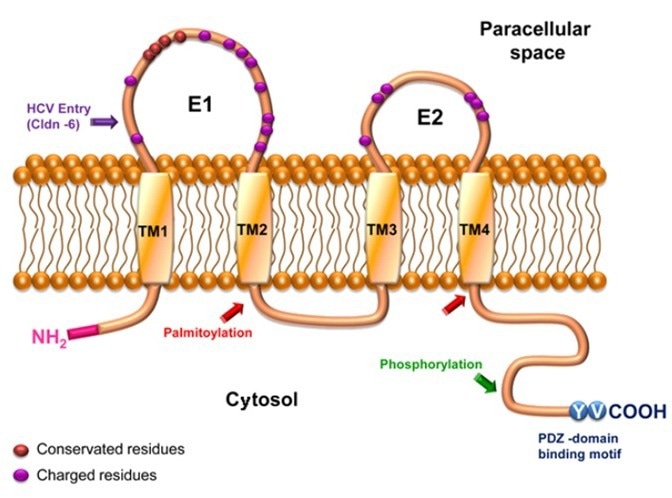
Diagram of Claudin 6 Structure. Image Credit: ACROBiosystems
STEAP1: A potential target for tumor immunology
The six-pass TP STEAP1 is primarily expressed on the surfaces of immune cells and tumor cells. STEAP1 has been shown to be highly expressed in a range of tumors and to be closely linked to tumor malignancy and prognosis.
STEAP1 is also involved in iron metabolism, playing a key role in tumor cells’ growth and proliferation.
These characteristics have seen STEAP1 emerge as a promising new target for tumor immunotherapy. A range of STEAP1-targeting antibody drugs and CAR-T cell therapies are currently under development, and these therapies could represent new strategies for tumor treatment.
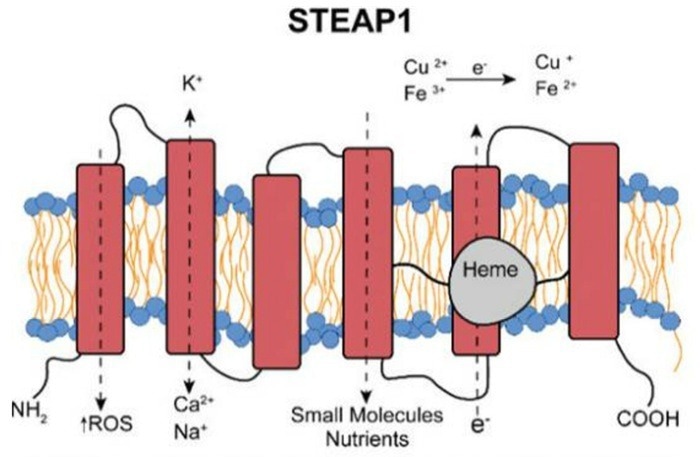
Diagram of STEAP1 Structure. Image Credit: ACROBiosystems
CCR8: A key target for tumor immunology
The seven-pass GPCR, CCR8, is highly and specifically expressed on tumor-infiltrating Tregs, while exhibiting very low expression in normal tissues and peripheral blood.
By targeting CCR8, it is possible to precisely modulate the immunosuppressive state within the tumor microenvironment.
The combination of CCR8-targeted drugs and PD-1 inhibitors has been shown to considerably improve antitumor activity, highlighting CCR8’s potential to become the next key target in the tumor immunosuppression field after PD-1.
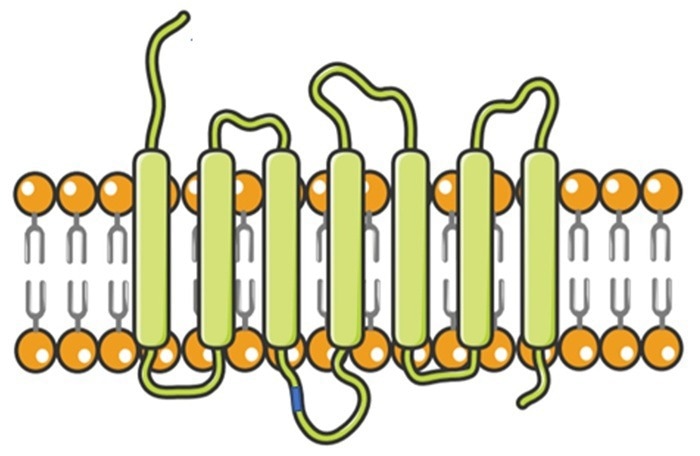
Diagram of CCR8 Structure. Image Credit: ACROBiosystems
Full-length active gallery: Solutions to overcome research challenges in preparing multi-pass TPs
The research path for multi-pass TPs is fraught with challenges despite its central role in a diverse array of physiological and pathological processes and its close links to various diseases.
The high structural complexity of multi-pass TPs makes it especially challenging to acquire full-length protein samples that can maintain their native conformation through traditional means.
The low expression of multi-pass transmembrane proteins, combined with their structural complexity and hydrophobicity, makes it challenging to produce target proteins with both high purity and high activity.
The functional validation of multi-pass TPs is also highly dependent on specific experimental conditions and advanced techniques.
These factors have led to relatively slow progress in the development of drugs targeting multi-pass TPs.
ACROBiosystems’ Full Length Active Gallery is a comprehensive platform-based solution designed specifically for the research and production of multi-pass TPs. It offers a stabilized structure and high activity required for effective drug development and mechanism studies.
Source: ACROBiosystems
| VLP Platform |
Detergent Platform |
Nanodisc Platform |
 |
 |
 |
- Higher abundance than that of overexpressing cells
- Can be used as the optimal targets for dendritic cells and phage display in vivo because of their 100-300 nm in size
- Suitable for immunization / ELISA / SPR / BLI / cell experiment / CAR detection
|
- Multi-pass TPs with complete conformation
- Can be accurately quantified
- Suitable for immunization / ELISA / SPR / BLI
|
- Full-length multi-pass TPs are in a natural membrane environment retaining high biological activity
- High hydrophilicity without detergents
- Suitable for immunization / ELISA / SPR / BLI / cell experiment / CAR detection
|
Hot molecules in full length active gallery
Source: ACROBiosystems
| . |
. |
. |
. |
. |
| CD20 |
Claudin 18.2 |
Claudin 18.1 |
Claudin 6 |
Claudin 9 |
| Claudin 4 |
GPRC5D |
CCR8 |
CCR5 |
CXCR4 |
| STEAP1 |
GLP-1R |
GCGR |
CD37 |
... |
References and further reading
- Thomas, B., Karuppiah Chockalingam and Chen, Z. (2023). Methods for Engineering Binders to Multi-Pass Membrane Proteins. Bioengineering, 10(12), pp.1351–1351. https://doi.org/10.3390/bioengineering10121351.
- Chen, J., et al. (2023). Targeting CLDN18.2 in cancers of the gastrointestinal tract: New drugs and new indications. Frontiers in Oncology, (online) 13, p.1132319. https://doi.org/10.3389/fonc.2023.1132319.
- Xu, M., et al. (2022). STEAP1–4 (Six-Transmembrane Epithelial Antigen of the Prostate 1–4) and Their Clinical Implications for Prostate Cancer. Cancers, (online) 14(16), p.4034. https://doi.org/10.3390/cancers14164034.
- Bar-Or, A., et al. (2021). Clinical Perspectives on the Molecular and Pharmacological Attributes of Anti-CD20 Therapies for Multiple Sclerosis. CNS Drugs, 35(9), pp.985–997. https://doi.org/10.1007/s40263-021-00843-8.
- Berenguer, J., et al. (2018). Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. 7(1), pp.1446660–1446660. https://doi.org/10.1080/20013078.2018.1446660.
- https://atlasgeneticsoncology.org/gene/50974/cldn6-%28claudin-6%29.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.