Cancer patients now have hope of a cure thanks to CAR-T cell therapy. In spite of its high efficacy, the toxicity known as cytokine release syndrome (CRS) has garnered popularity.
The goal of this article is to summarize the vital points of CRS assessment, grading and management in CAR-T Therapy. The article also provides answers to the following questions:
- What are the clinical signs and symptoms of CRS?
- What is the management of the CRS?
- What is the consensus grading of the ASTCT CRS?
- What is the best way to manage CRS with tocilizumab?
Assessment, grading and management of CRS in CAR-T Therapy
CAR-T cell therapy has given many cancer patients hope of a cure in recent years.
Despite its high efficacy, the toxicity known as cytokine release syndrome (CRS) is a serious issue. T lymphocytes are activated and proliferate rapidly after receiving CAR-T, resulting in the excessive release of cytokines such as IFN-γ, TNF-α, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10 and IL-12.
Possible side effects include fever, myalgia, hypotension, dyspnea, blood coagulation disorders and end-organ dysfunction. These can induce permanent damage or even death.
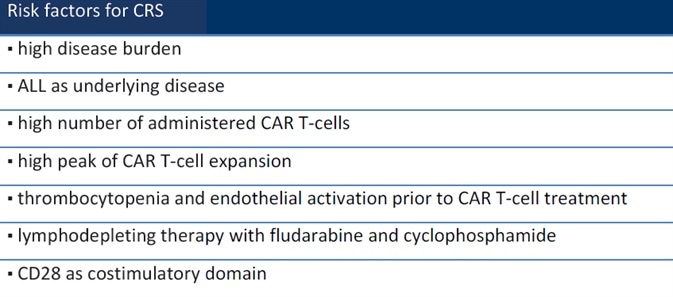
CRS, in a nutshell, is an acute systemic inflammatory syndrome linked to CAR-T cell therapy.1 Several factors, including disease state, treatment procedure and drugs, can influence the likelihood of developing CRS (Table 1).2
Table 1. Risk factors for CRS. Source: ACROBiosystems
When attempting to identify the onset of CRS in a clinical setting, it is possible to look at serum CRP and ferritin levels in the blood as they are usually elevated in CRS patients. They can be used as biomarkers to determine if CRS has occurred.
However, all of these markers — CRP, ferritin, AST, LDH, ALT and creatinine — appear only after CRS symptoms have manifested, and it is not possible to determine the onset of CRS using them.
The majority of the hospitals are unable to monitor cytokine levels in real-time; instead, they can only look at the clinical signs and symptoms. When CRS occurs, it is usually determined by factors such as the type of CAR-T therapy used and the patient’s disease state.
The CRS peaks on the 3rd and 7th days after the administration of Kymriah, which contains the costimulatory domain of 4-1BB (CD137).
The CRS occurs earlier on the second day of administration for patients taking Yescarta or Tecartus, which contain the costimulatory domain of CD28. However, some delayed CRS has been reported after three weeks of CAR-T administration.2
The American Society for Transplantation and Cell Therapy (ASTCT) published a consensus grading guideline for CRS in 2019 (Table 2). Fever (≥38 °C) is a requirement, according to the guidelines.
The conditions worsen with or without hypotension and/or hypoxia. The ASTCT grading system has been shown in studies to provide a clinically accurate, objective and repeatable assessment of the severity of CRS.2–3
Table 2. ASTCT CRS Consensus Grading. Source: ACROBiosystems
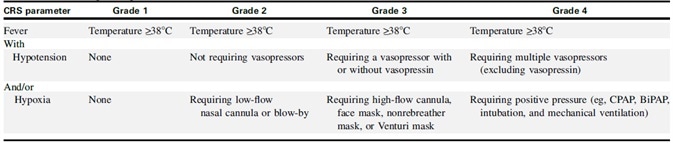
Grade 1: Patients with CRS who have a fever and some non-specific signs but no hypotension or hypoxia are classified as grade 1 patients. Antipyretic drugs are infused intravenously as part of the treatment strategy.
Grade 2: Grade 2 patients are those who have a fever (≥38.0 °C) with hypotension that does not require vasopressors and/or hypoxia that necessitates oxygen delivery via a low-flow nasal cannula (≤ 6 L/minute) or blow-by.
However, due to vasodilation and capillary leakage, CRS patients are at risk of developing edema. Fluid infusion must, therefore, be closely monitored. For grade 2 patients, tocilizumab, an IL-6 inhibitor, can be used. Early use of tocilizumab has been shown in studies to reduce the risk of severe CRS and organ failure while preserving the efficacy of CAR-T therapy.
Grade 3: Patients with Grade 3 CRS — patients with fever, low blood pressure, and no response to fluid infusion, as well as patients who require high flow oxygen (≥ 6 L/minute) — should be taken care of in the intensive care unit (ICU) and treated with Belizumab and glucocorticoids (dexamethasone, etc.).
It is recommended to use a vasopressor (such as norepinephrine) and/or a high-flow nasal cannula (>6 L/minute) to prevent further deterioration and visceral damage. Furthermore, as high-grade CRS patients are prone to cardiac dysfunction, it is recommended that their cardiac function be closely monitored.
Grade 4: Grade 4 CRS patients have a fever of ≥38.0 °C with hypotension requiring multiple vasopressors (excluding vasopressin) and/or hypoxia requiring positive pressure (for example, CPAP, intubation, bilevel positive airway pressure, mechanical ventilation) not attributable to any other cause.
Methylprednisolone, at a dose of 1 g per day, is recommended in addition to the treatment for grade 3 CRS. High-grade CRS is shown to be reversible with early intervention.
Tocilizumab (Actemra) a drug from Roche was approved by the FDA in 2017. It was developed to treat CRS induced by CAR-T therapy by blocking the inflammatory cytokine interleukin-6 (IL-6).
Tocilizumab clinical guidelines:4
- Tocilizumab should be considered in the early stages of CRS for elderly patients or patients with severe complications
- Adults with grade 2 CRS and children with grade 3 CRS can receive tocilizumab
- Tocilizumab can be given to pediatric patients with grade 2 CRS who are fever-intolerant
- If the conditions do not improve after the first dose of tocilizumab, the second dose should be administered along with glucocorticoids. Anakinra, Siltuximab and a high dose of methylprednisolone should be considered if the condition does not improve.
Researchers are making big efforts to help prevent or treat CRS. Nature published two articles on the same day in 2018 about a new way to control CAR-T toxicity. Inhibition of IL-1 reduces macrophage-induced CRS, according to their findings.
Michel Sadelain, director of the Sloan Kettering Cancer Center’s Cell Engineering Center, published an article in Science Translational Medicine in 2019. In mouse models, he discovered a functional switch in CAR-T cells that helped them avoid CRS.5
A research team from the Chinese Academy of Medical Sciences published an article in the journal Science Immunology in January 2020. The mechanism of CRS and the process of pyrolysis caused by CAR-T therapies were discussed in this article.
The entire process of CRS triggered by CAR-T cell therapy can be visualized in their clear microscope images.6 Breakthroughs in CAR-T therapies and CRS are anticipated in the near future, thanks to ongoing clinical research.
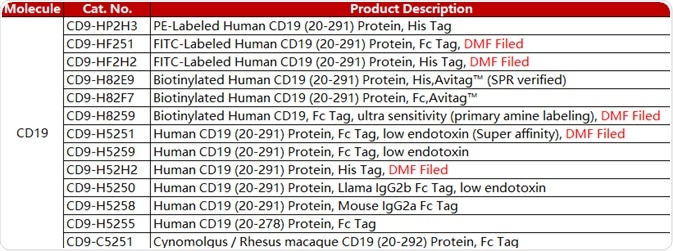
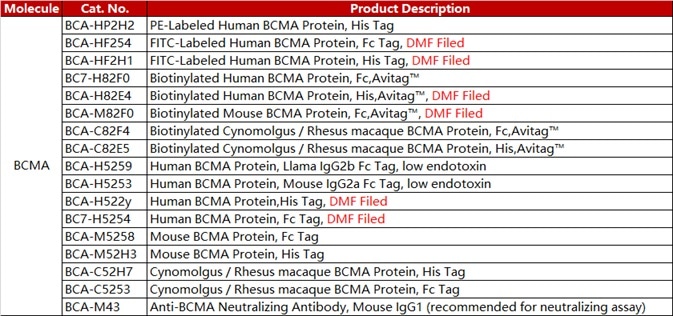
ACROBiosystems designed a series of CAR-T target proteins — CD19, BCMA, CD22 MSLN and EGFR. The company provides non-labeled, fluorescent-labeled, biotin-labeled and other forms of these proteins. It also provides flow cytometry protocols for detecting CAR expression.
In anti-CD19 CAR detection assays, the ACRO FITC-labeled CD19 protein (Cat. No. CD9-HF2H2) is highly specific and sensitive. In addition, an antibody (Cat. No. FM3-FY45) that targets the antigen-binding epitope of FMC63 scFv was also created. This product provides a new method for detecting Anti-CD19 (FMC63) CARs.
The FITC-labeled BCMA protein from ACRO (Cat. No. BCA-HF254) has excellent sensitivity. This product is used widely in various assays and is well-known in the industry.
Product list
CD19
Table 3. Source: ACROBiosystems
BCMA
Table 4. Source: ACROBiosystems
Anti-FMC63 mAb
Table 5. Source: ACROBiosystems
Case study
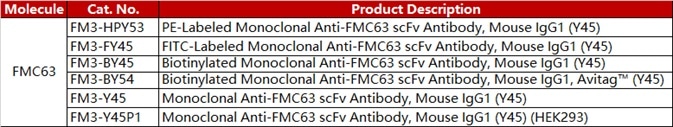
1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was used as a negative control.
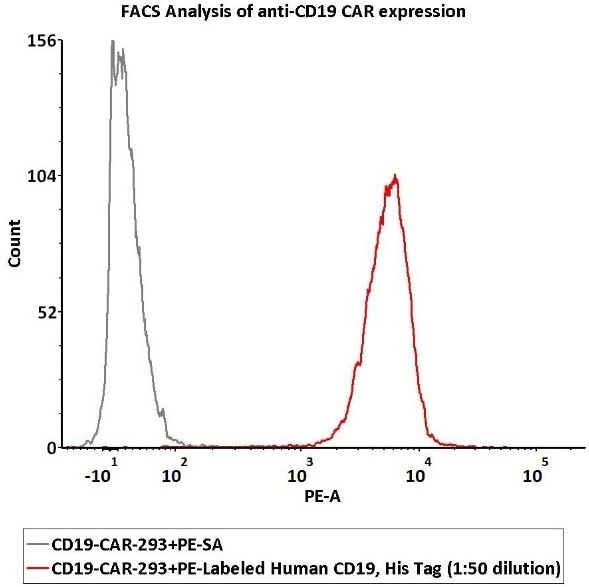
Image Credit: ACROBiosystems
2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control, respectively. The binding activity was evaluated with the FITC signal.
Image Credit: ACROBiosystems
1e6 of the Anti-BCMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No. BCA-HP2H2) and negative control protein, respectively. The binding activity was evaluated with PE signal.
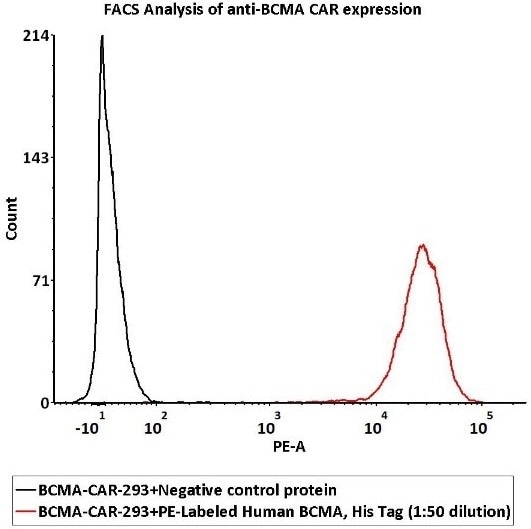
Image Credit: ACROBiosystems
References
- https://www.cn-healthcare.com/articlewm/20190306/content-1047067.html.
- Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34-48.
- Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146(5):940-948.
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511.
- Mestermann, Katrin, et al. "The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells." Science translational medicine 11.499 (2019).
- Liu, Yuying, et al. "Gasdermin E–mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome." Science immunology 5.43 (2020).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.