Within cancer immunotherapies, immune checkpoint inhibitors represented a breakthrough in both the clinical approval and developmental direction of antibody therapies.
The specificity of antibodies provided healthcare providers with a highly effective cancer therapy that minimized the unwanted side effects of chemotherapies. However, the number of patients that were non-responsive, relapsed, or refractive towards immune checkpoint inhibitors was a major limitation towards its adoption as a therapy.
This naturally meant that scientists began to search for alternative strategies, taking advantage of research into the immunoregulatory network from the discovery of checkpoint inhibitors.
Rather than modulating the immune regulatory network, the primary alternative strategy was to use immune agonistic antibodies (IAAs) to directly activate the immune cells. IAAs are therefore designed to enhance the immune response against cancer cells, leading to immune cell survival and increased tumor cell cytotoxicity.
As outlined below, there are several key factors of IAAs that assist in their potential to combat various cancers.
The agonistic antibodies of CD40
A member of the tumor necrosis factor receptor (TNFR) superfamily, CD40, is expressed on antigen-presenting cells. This includes macrophages, dendritic cells, and B cells. CD40, therefore, plays a crucial role in the immune system and allows the dendritic cells to activate antigen-specific T cells at the same time as promoting B cell activation and proliferation in addition to antigen presentation.
Antibodies targeting CD40 are designed to mimic the effects of CD40’s natural ligand, CD40L, and to activate the signaling pathway. This activation results in the maturation of dendritic cells, which activate and expand the antigen-specific T cells. APX005M, ChiLob7/4, ADC_1013, SEA-CD40, and selicrelumab are several of the current therapeutic antibodies targeting CD40.
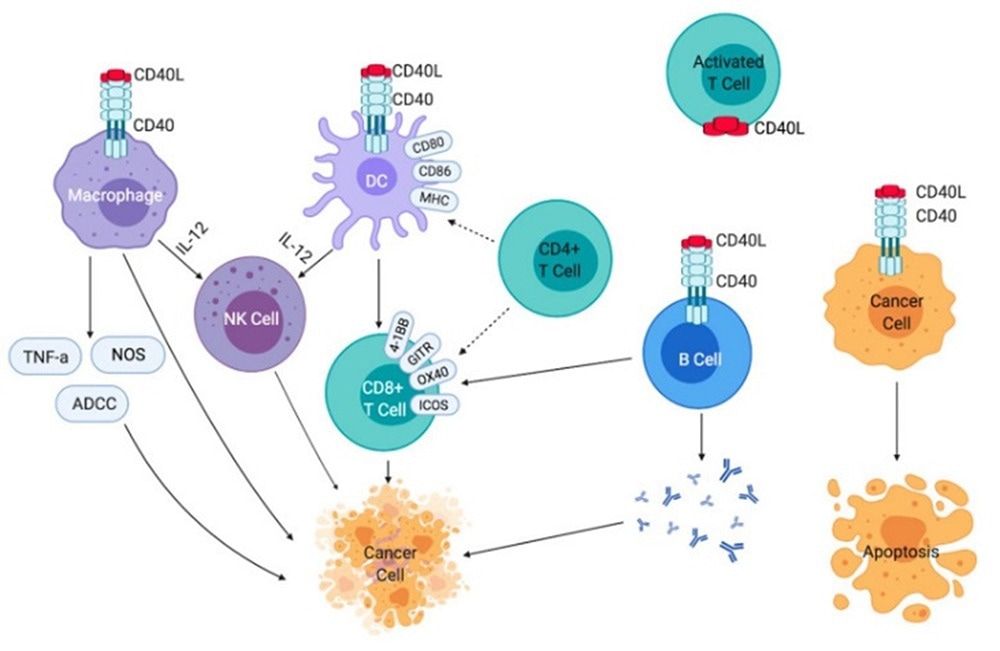
Effects of CD40-CD40L signaling on immune cells and cancer cells.2 Image Credit: ACROBiosystems
However, several key challenges are involved in developing CD40-targeting IAAs, the first of which is toxicity.
CD40 is highly expressed across numerous cell types, thus leading to systemic inflammation throughout the body as a result of the increased immune response due to CD40 being highly expressed across numerous cell types. Certain CD40 IAAs have, therefore, reported low levels of antitumor efficacy and immune activation, which further emphasizes the need for personalized dosing to balance efficacy with toxicity.
To enhance the binding of CD40-targeting IAAs, researchers have explored other developmental strategies to address these challenges, such as Fc engineering. CD40 IAAs promote a higher order of crosslinking between the antibody and neighboring cells by improving the binding affinity to FcγRIIB. This, therefore, enhances the clustering and signaling of CD40 around the target cell.
The co-administration of therapies, including other immune-based therapies and anti-angiogenic antibodies, is another more common strategy (such as checkpoint inhibition, colony-stimulating factor 1 receptor (CSF-1R) inhibitors, and TLR3 agonists).
In preliminary studies, this has shown to be effective: combining CD40 agonist antibodies CP-870,893 with immune checkpoint inhibitor tremelimumab elicited a response rate of 27% in patients with advanced melanoma.1
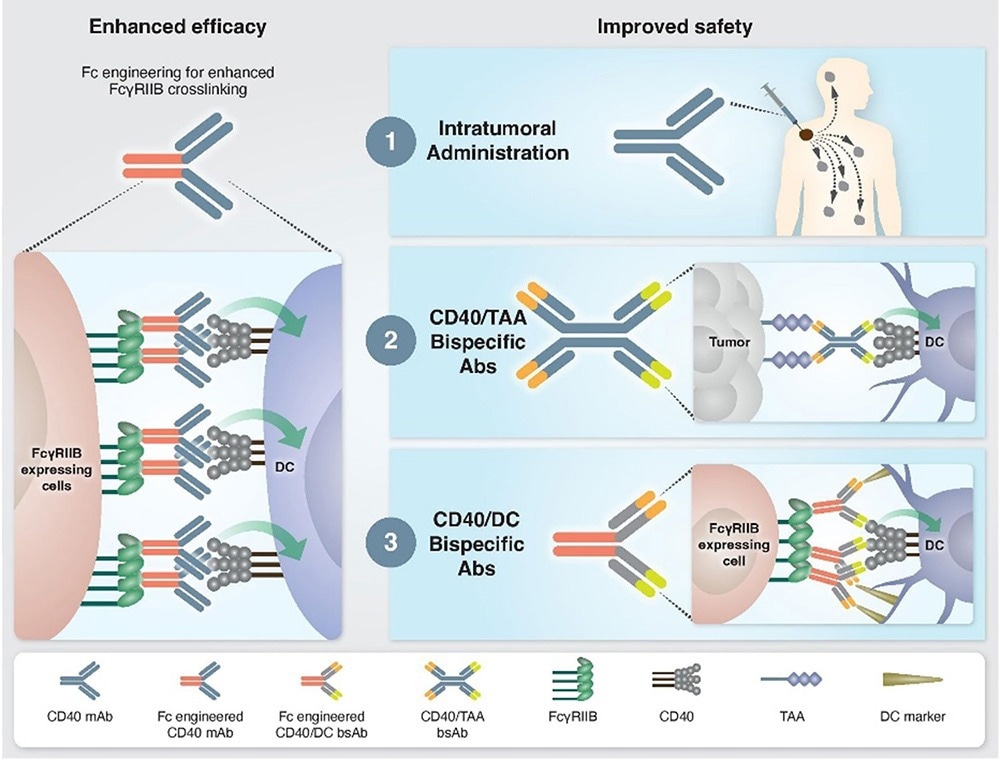
Approaches to enhance the efficacy and safety of CD40 agonistic mAbs.1 Image Credit: ACROBiosystems
Overall, CD40 IAAs have immense potential to overcome the challenges of checkpoint inhibitors. However, suboptimal efficacy and toxicity still need to be addressed, and combination therapy with other immunotherapies should be investigated to enhance the efficacy of CD40 agonistic antibodies.
OX40 and its agonistic antibodies
Also known as TNFRSF4 or CD134, OX40 is a co-stimulatory receptor belonging to the TNF superfamily found on a T cell’s surface.3 As the only ligand of OX40, OX40L is mainly expressed on activated antigen-presenting cells (APCs).3
T cell activation, proliferation, and survival are all enhanced when OX40 is activated by its natural ligand, OX40L, which leads to a stronger immune response against cancer cells.
Moreover, high OX40 expression in the tumor immune infiltration has been used prognostically for several types of cancer.3 Therefore, OX40 is widely regarded as one of the most promising and ideal targets for novel cancer immunotherapy.
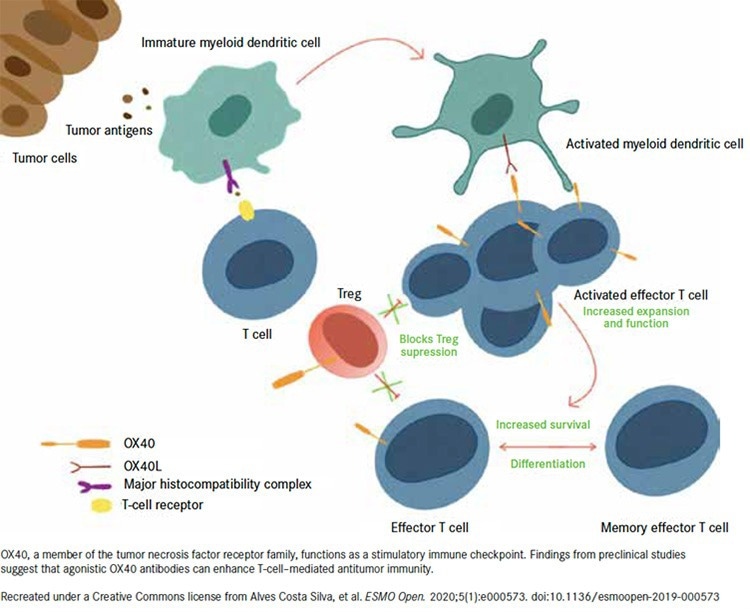
OX40 Mechanisms of Action.5 Image Credit: ACROBiosystems
Various agonistic antibodies targeting OX40 have been developed and evaluated in early clinical trials, such as 9B12/ MEDI6469, MEDI-0562 (developed by AstraZeneca), 11D4 (PF-04518600, developed by Pfizer), and MOXR0916(developed by Roche).4,5
However, as monotherapy, OX40 agonists have failed to live up to expectations, and indeed due to modest antitumor activity, several have been withdrawn.5
There is still a great deal of interest in the potential of OX40-targeted drugs despite such setbacks, but this interest has focused primarily on combination therapy, especially with immune checkpoint inhibitors based on a preclinical demonstration of synergy. Preliminary toxicity testing has been completed by Roche’s humanized effector-competent OX40 agonist antibody RG7888 (MOXR0916/pogalizumab).
This has taken place in combination with the PD-L1 inhibitor atezolizumab in patients with advanced solid malignancies, and the result was good toleration of the combination with no dose-limiting toxicities.4 In other therapies, the application of co-stimulatory anti-OX40 mAbs as adjuvants is being explored in several clinical trials, such as anti-OX40, combined with radiotherapy and chemotherapy.4
Various important considerations, such as epitope selection, binding affinity, receptor occupancy, and valency, must all be taken into account when designing the optimal OX40 agonist antibodies. One common challenge that occurs throughout the development of agonist antibodies is that searching for the highest tolerable dose of receptor agonists has the potential to exhaust T cells and negate any antitumor efficacy.5
Additionally, designing an effective OX40 agonist antibody must be able to reproduce this effect, given that co-stimulatory receptor clustering is critical in signal activation, commonly induced by native ligand binding.5
However, achieving this effect through enhancement of FcγR binding has resulted in the depletion of T cells due to another co-stimulatory agonist antibody. An alternative strategy explored previously is to increase valency.
Inhibrx is developing INBRX-106, a hexavalent OX40 monoclonal antibody that can bind 6 OX40 receptors per molecule of drug.
Bispecific antibodies, designed to engage two different targets, are also being analyzed, such as ATOR-1015 targeting OX40 and the coinhibitory receptor CTLA-4 or F-star Therapeutics’ FS120, a bispecific antibody that targets a second co-stimulatory receptor, 4-1BB (CD137), in addition to OX40.
Shattuck Labs is pursuing a different drug design to achieve a similar effect to these bispecific antibodies, with its Agonist Redirected Checkpoint platform to create SL-279252, a bifunctional fusion protein that comprises the extracellular domains of OX40L and PD-1 joined by a central Fc domain.5
4-1BB and its agonistic antibodies
4-1BB, otherwise known as CD137, is a co-stimulatory receptor expressed on the surface of activated T and B cells, monocytes, macrophages, dendritic cells (DCs), regulatory T cells (Tregs), and natural killer (NK) cells.
CD137 can interact with TNFR-associated factor (TRAF) proteins, including TRAF1, TRAF2, and TRAF3, which activate different signaling pathways such as nuclear factor κB (NF-κB), MAPK, ERK, and JNK.4
The upregulation of survival genes, such as Bcl-XL and Bfl-1, the downregulation of proapoptotic molecules like BIM, and the transmission of signals that stimulate cell division are all promoted by the activation of the NF-κB pathway.
Additionally, biochemical signals are triggered by the 4-1BB/4-1BB ligand (4-1BBL) signaling, which increases TH1 cytokines, including interleukin 6 (IL-6), IL-8, TNF, IL-12, and interferon γ (IFN-γ), as well as suppress TH2 cytokines, potentiate activation, survival, proliferation, and cytotoxicity of T cells, increase IL-2 production, and also promote the maturation of DCs.4
Several 4-1BB agonistic antibodies are currently under investigation in clinical trials for the treatment of various types of cancer, including urelumab (BMS-663513), PF-05082566, and utomilumab. However, their efficacy was limited, thus the clinical development of utomilumab was ultimately discontinued.6
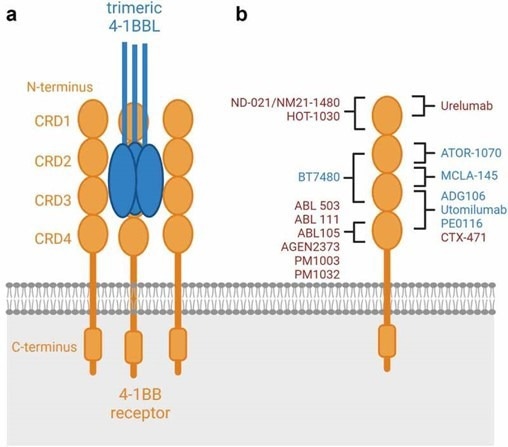
Schematic binding epitopes to the 4–1BB receptor.6 Image Credit: ACROBiosystems
The first group of second-generation 4-1BB agonists comprises a human IgG1 or IgG4 scaffold and follows the mechanism of action of first-generation agonistic antibodies. These agonists are highly dependent on FcγR crosslinking, yet, they bind to different epitopes.
A second group of 4-1BB agonists were designed to be polyvalent with bi-, tri- or tetra=- specific 4-1BB, thus allowing for a more comprehensive binding of 4-1BB while mediating 4-1BB hyperclustering. As such, These bi-, tri-, or tetra-specific agonists can display agonistic or inhibitory activity, leading to further anti-tumoral properties.6
Researchers from Roche recently published a study titled “A first-in-human study of the fibroblast activation protein-targeted, 4-1BB agonist RO7122290 in patients with advanced solid tumors” in the journal Science Translational Medicine.
The study evaluated the efficacy and safety of RO7122290 in patients with metastatic or advanced solid tumors. The study found that only three dose-limiting toxicities were reported, and the drug was generally well-tolerated.
Moreover, a complete or partial response was experienced by eleven patients, highlighting the potential of RO7122290 for the treatment of solid tumors in combination with atezolizumab or other immune-oncology agents. However, it must be noted that to confirm its efficacy and safety profile, further evaluation of RO7122290 is needed.7
Summary
T-cell agonists, among other immune co-stimulatory agonists, have demonstrated great promise in cancer immunotherapy. Overall, it is fair to state that the majority of antibodies have demonstrated promising results in early-phase trials, while some agents have failed to fulfill their intended goals.
When it comes to inhibiting tumor progression, combination therapy trials involving checkpoint inhibitors and radiotherapy or chemotherapy have also shown potential synergistic effects.
However, important challenges need to be addressed in finding ways to manage immune-related adverse events, discover potential biomarkers to predict benefits from immune-agonist treatment, and determine optimal treatment delivery methods.
Despite these challenges, T-cell agonists are likely to be an important component of future cancer immunotherapy and are worth the effort to monitor its research progress further.7
References and further reading
- Salomon Ran., Dahan Rony.(2022). Next Generation CD40 Agonistic Antibodies for Cancer Immunotherapy. Front Immunol, 13(undefined), 940674. doi:10.3389/fimmu.2022.940674
- Djureinovic Dijana., Wang Meina., Kluger Harriet M.(2021). Agonistic CD40 Antibodies in Cancer Treatment. Cancers (Basel), 13(6), undefined. doi:10.3390/cancers13061302
- Zhang Jing., Jiang Xiaoyong., Gao Han., Zhang Fei., Zhang Xin., Zhou Aiwu., Xu Ting., Cai Haiyan.(2022). Structural Basis of a Novel Agonistic Anti-OX40 Antibody. Biomolecules, 12(9), undefined. doi:10.3390/biom12091209
- Mascarelli Daniele E., Rosa Rhubia S M., Toscaro Jessica M., Semionatto Isadora F., Ruas Luciana P., Fogagnolo Carolinne T., Lima Gabriel C., Bajgelman Marcio C.(2021). Boosting Antitumor Response by Co-stimulatory Strategies Driven to 4-1BB and OX40 T-cell Receptors. Front Cell Dev Biol, 9(undefined), 692982. doi:10.3389/fcell.2021.692982
- Fresh Approaches May Be Key to Unlocking OX40 Checkpoint
- Claus Christina., Ferrara-Koller Claudia., Klein Christian.(2023). The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy. MAbs, 15(1), 2167189. doi:10.1080/19420862.2023.2167189
- Melero Ignacio., Tanos Tamara., Bustamante Mariana., Sanmamed Miguel F., Calvo Emiliano., Moreno Irene., Moreno Victor., Hernandez Tatiana., Martinez Garcia Maria., Rodriguez-Vida Alejo., Tabernero Josep., Azaro Analia., Ponz-Sarvisé Mariano., Spanggaard Iben., Rohrberg Kristoffer., Guarin Ernesto., Nüesch Eveline., Davydov Iakov I., Ooi Chiahuey., Duarte José., Chesne Evelyne., McIntyre Christine., Ceppi Maurizio., Cañamero Marta., Krieter Oliver.(2023). A first-in-human study of the fibroblast activation protein-targeted, 4-1BB agonist RO7122290 in patients with advanced solid tumors. Sci Transl Med, 15(695), eabp9229. doi:10.1126/scitranslmed.abp9229
- Choi Yeonjoo., Shi Yaoyao., Haymaker Cara L., Naing Aung., Ciliberto Gennaro., Hajjar Joud.(2020). T-cell agonists in cancer immunotherapy. J Immunother Cancer, 8(2), undefined. doi:10.1136/jitc-2020-000966
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.