SARS-CoV-2 is slowly mutating as it continues its spread. Virologists call it the D614G mutation: at the 614th amino-acid position of the spike protein,ce the amino acid aspartate (D, in biochemical shorthand) was routinely being supplanted by glycine (G).
Since the end of February 2020, this mutant increasingly inhabits a proportion of variants detected, which makes it the most commonly identified variant in various parts of the world at present. This key attribute has grabbed the attention of researchers ready to further investigate if the D614G mutation is more transmissible/infectious or more deadly?
Recently, Cell published a research article titled “Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant”. Scientists examined the D614G mutation from various aspects of structural biology and cell biology. This article outlines some of the main points.
As of June 25, 2020, the global frequency of the S protein D614G variant has steadily increased over time and is now present in almost 74% of all published variants, according to the GISAID SARS-CoV-2 database. (Fig. 1)
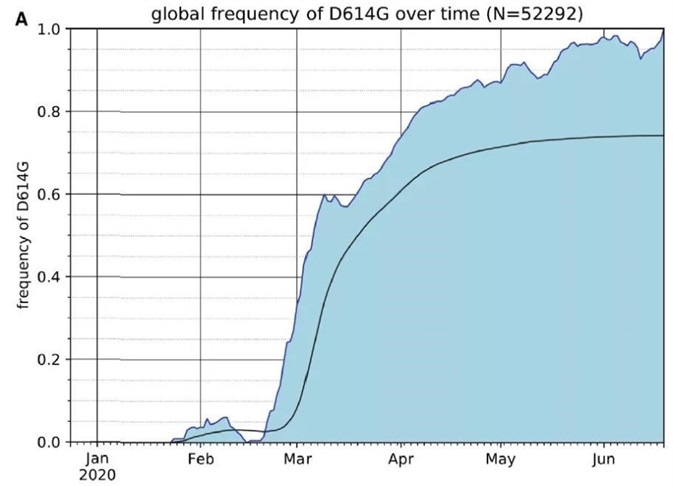
Figure 1. The Frequency of the SARS-CoV-2 S Protein D614G Variant over the Course of the Pandemic Has Increased Nearly to Fixation. Image Credit: ACROBiosystems
In order to contrast the ability of the ancestral S protein D614 or the D614G variant to mark virions for infection, construction of the pseudotyped lentivirus was performed. Various cell models are examined, including Calu-3 human lung epithelial cells, HEK293 cells, Caco-2 human colon epithelial cells, and SupT1 cells. The data illustrates in Fig. 2, the infectivity of D614G is 4 to 9 times greater than D614.
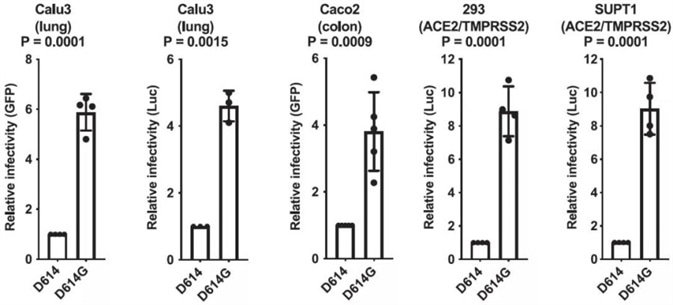
Figure 2. SARS-CoV-2 D614G S Protein Variant Enhances Infectivity of Pseudotyped Lentiviruses in Cell Culture. Image Credit: ACROBiosystems
For SARS-CoV-2 to effectively penetrate human cells, the spike protein present on the virus surface must bind to the host receptor protein, ACE2. While D614G is situated outside of the receptor-binding domain, this mutation could transform ACE2-binding properties via allosteric effects.
As established by the surface plasmon resonance (SPR) results, as shown in Fig. 3, D614G reduces the affinity for ACE2 by intensifying the rate of dissociation. The data demonstrates that the enhanced infectivity of D614G cannot be explained by greater ACE2 binding strength.
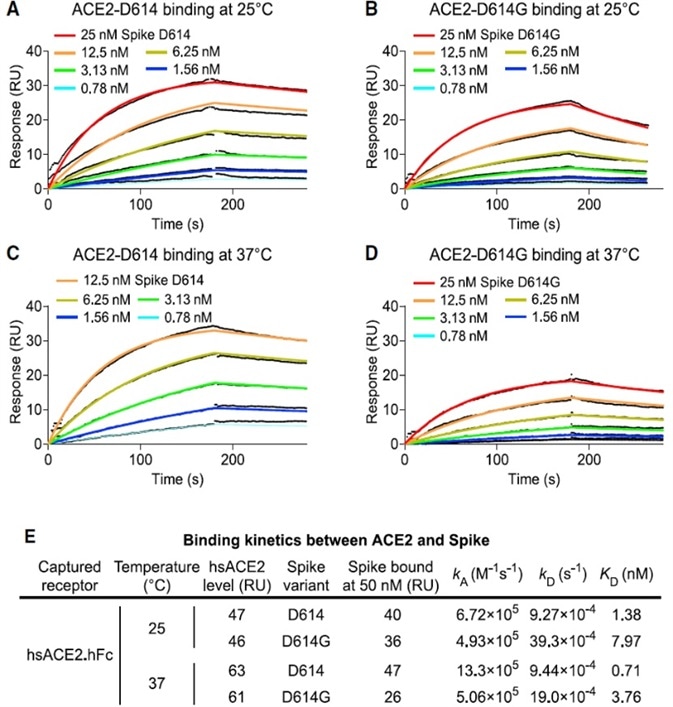
Figure 3. SARS-CoV-2 D614G S Protein Variant Binds ACE2 Weaker than the Ancestral Protein. Image Credit: ACROBiosystems
Consequently, a majority of the COVID-19 therapeutic antibodies currently in development target the receptor-binding domain of spike protein. While this structural change is non-conservative, it queries whether it will jeopardize the effectiveness of these therapeutic antibodies currently being developed: some studies were conducted using Regeneron antibodies.
The results of these studies are outlined below in Fig. 4, suggesting D614G and the ancestral D614 are uniformly sensitive to these neutralizing antibodies targeting the receptor-binding domain.
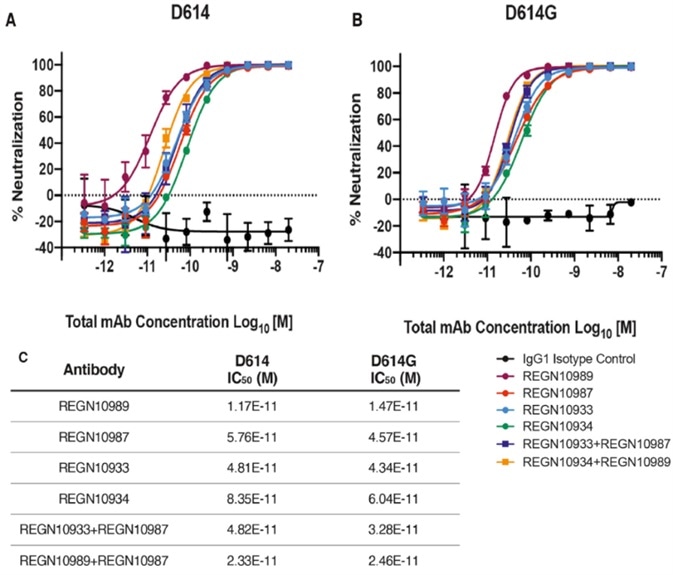
Figure 4. Neutralization Potency of Monoclonal Antibodies Targeting the SARS-CoV-2 S Protein Receptor-Binding Domain Is Not Attenuated by D614G. Image Credit: ACROBiosystems
Principally, SARS-CoV-2 spike trimer protein exhibits two conformational states. The “up” characterizing a receptor-accessible state, and the “down” characterizes a receptor-inaccessible state (shown in Fig. 5) – the “up” state accounts for 54% while the “down” state accounts for 46%.[2]
![Spike protein receptor binding domain binding to ACE2 for viral entry [3].](https://www.news-medical.net/image-handler/picture/2020/12/art2-5.jpg)
Figure 5. Spike protein receptor-binding domain binding to ACE2 for viral entry [3]. Image Credit: ACROBiosystems
To further investigate alteration in conformation as a result of D614G mutation, researchers conducted Cryo-EM testing. They discovered that there was no considerable change in the S2 subunit. However, there was a variation in the D614G S1 subunit, leading to the subsequent receptor binding domain conformation change (Fig. 6). It is clear to see that there is up to 95% “up” state conformation in D614G, which essentially increases the chance of infection.
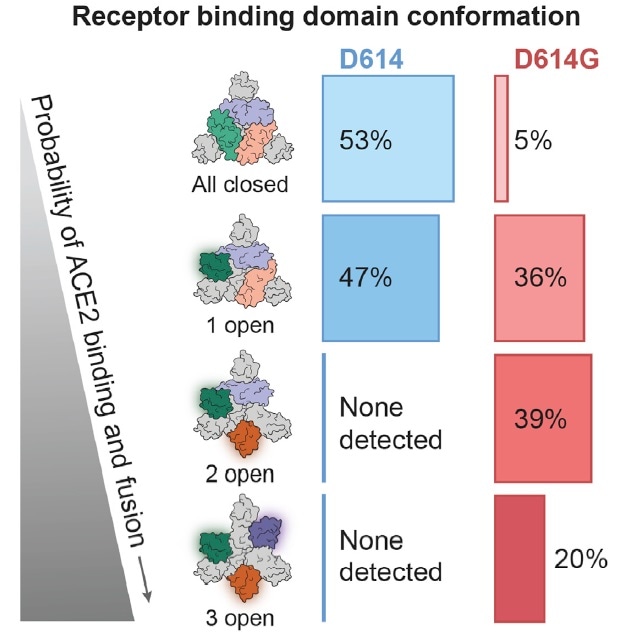
Figure 6. D614G mutation increased the amount of RBD 2 open and 3 open. Image Credit: ACROBiosystems
As it stands, around 200 COVID-19 vaccine development studies are ongoing. When assessing the efficacy of the vaccine, researchers and scientists typically use spike protein or receptor binding domains to determine the antibody titer. It should be mentioned that Moderna used the D614G spike protein instead in these assays.[5]
Trimeric spike protein (D614G) products from ACROBiosystems
ACROBiosystems developed several stable recombinant spike trimer protein products such as ancestral D614G spike trimer protein and D614G spike trimer protein, as well as relevant protein-coupled magnetic beads, all of which are designed for advances in COVID-19 vaccine and therapeutic development.
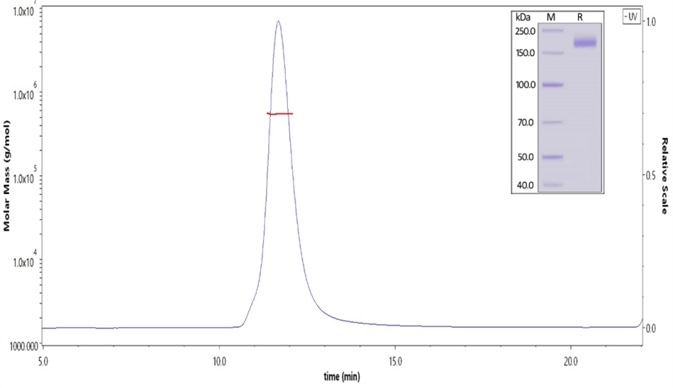
Figure 1. The purity of SARS-CoV-2 S protein (D614G), His Tag, Super stable trimer (Cat. No. SPN-C52H3) was more than 90% and the molecular weight of this protein is around 520-620 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
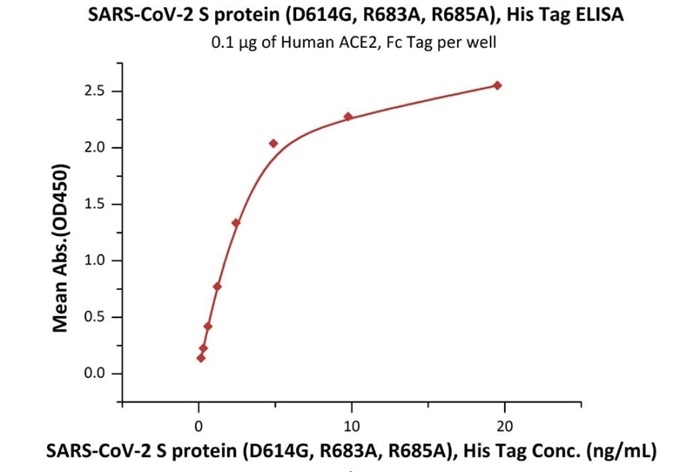
Figure 2. Immobilized Human ACE2, Fc Tag (Cat. No. AC2-H5257) at 1 μg/mL (100 μL/well) can bind SARS-CoV-2 S protein (D614G), His Tag, Super stable trimer (Cat. No. SPN-C52H3) with a linear range of 0.2-5 ng/mL. Image Credit: ACROBiosystems
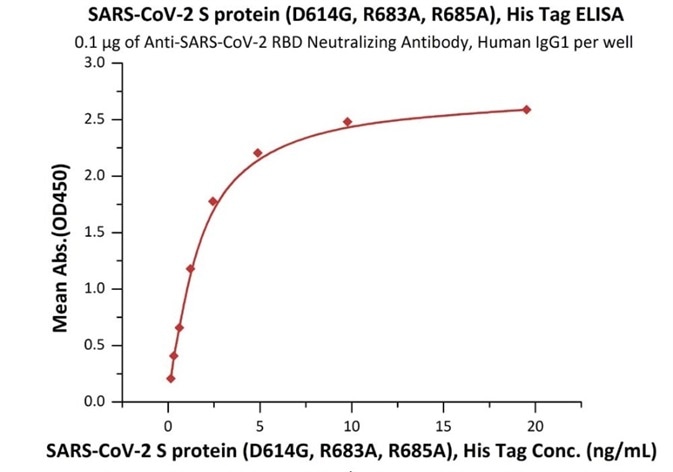
Figure 3. Immobilized Anti-SARS-CoV-2 RBD Neutralizing Antibody, Human IgG1 (Cat. No. SAD-S35) at 1 μg/mL (100 μL/well) can bind SARS-CoV-2 S protein (D614G), His Tag, Super stable trimer (Cat. No. SPN-C52H3) with a linear range of 0.2-2 ng/mL. Image Credit: ACROBiosystems
References and Further Reading
- Structure-based design of prefusion-stabilized SARS-CoV-2 spikes.
- Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation.
- Cell entry mechanisms of SARS-CoV-2 .
- Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy.
- Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults.
About ACROBiosystems
ACROBiosystemsis a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.