
Image Credit: ACROBiosystems
Beware of the atopic march: From skin to systemic disease
September 14 was the eighth World Atopic Dermatitis Day. Atopic dermatitis (AD) is a common, chronic, and recurrent inflammatory skin disease characterized by severe pruritus and eczematous lesions.
As the first stage in the ‘atopic march’, AD is linked to fundamental pathological alterations, including a compromised skin barrier, immune dysregulation, and microbiome imbalance.
Understanding AD is vital due to its strong connection with other allergic conditions. Studies indicate that around 30 to 50% of individuals with moderate-to-severe AD will develop additional atopic conditions such as allergic rhinitis (AR) and allergic asthma (AA), hallmarks of the typical atopic march.
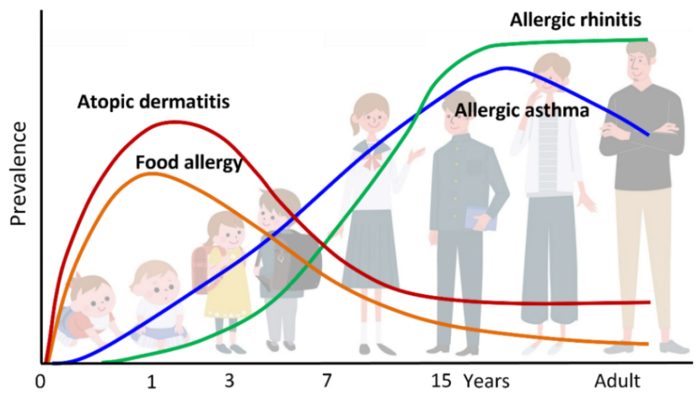
AD and the Atopic March. Image Credit: ACROBiosystems
The "atopic march" describes the age-related progression of allergic diseases, presenting as the sequential onset of atopic disorders propelled by evolving allergen sensitization, impacting multiple organ systems. AD is often the earliest manifestation, followed by IgE-mediated food allergy (FA), AR, and AA.
AD prevalence in children ranges from 17% to 24%. Among affected individuals, 48% develop symptoms before six months of age and 89% before age five, with incidence peaking at around two years. Approximately half of patients experience remission by adolescence, while the remainder continue into adulthood. AD severity strongly correlates with elevated risk for subsequent FA, AR, and AA.
To support AD research, ACROBiosystems has established an extensive product portfolio featuring highly active recombinant proteins, stable cell lines, and inhibitor screening kits.
Its solutions support the entire therapeutic development process, including target identification and validation, candidate drug screening and development, and CMC production and quality control. By supporting each stage, they accelerate the efficient translation of novel AD treatments from major studies to clinical application.

Image Credit: ACROBiosystems
Why does AD function as the initiator of the atopic march?
AD pathogenesis follows a distinct stepwise progression. It often originates in regions of skin that look clinically 'normal' but exhibit substantial barrier impairment, allowing environmental allergens to penetrate the epidermis.
These antigens are captured by epidermal Langerhans cells (LCs) and dermal dendritic cells (DCs), inducing an immune response that recruits and activates inflammatory cells locally.
In addition, barrier disruption prompts keratinocytes to release epithelial alarmins, including TSLP and IL-33, as well as chemokines such as CCL17. These mediators intensify itch signaling and activate both type 2 innate lymphoid cells (ILC2s) and Th2 cells, establishing an early immune environment dominated by type 2 inflammation.
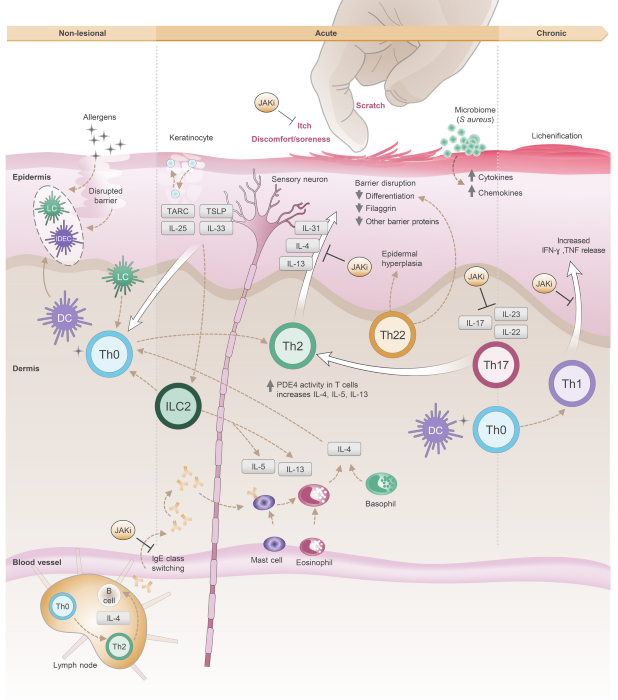
Mechanistic Insights into AD. Image Credit: ACROBiosystems
During the acute phase of AD, Th2 cells secrete IL-4 and IL-13, driving IgE class switching, weakening barrier proteins, and recruiting eosinophils and other inflammatory cells. This amplifies inflammation and increases vulnerability to infection.
As the disease advances to the chronic phase, the immune response shifts to a mixed Th1/Th2 profile, with additional dysregulated cytokines such as IL-17, IL-22, and IL-23. This promotes a self-perpetuating cycle of chronic inflammation, structural remodeling of the skin, and persistent pruritus.
The multi-stage, multi-pathway, and multi-target immune dysregulation explains why AD serves as the initiating factor in the atopic march, increasing the risk of subsequent allergic diseases.
Targeted breakthroughs: A new era in AD treatment
In recent years, biologics and small-molecule targeted treatments have propelled a new surge of development in AD therapies, with multiple agents already approved and achieving remarkable market success.
Dupilumab, the first fully human monoclonal antibody targeting IL-4Rα, has dominated the worldwide AD market since its approval in 2017, generating over $14.2 billion in sales in 2024 alone.
Tezepelumab, the first monoclonal antibody against TSLP, was initially approved for asthma treatment, but is now being evaluated for potential use in AD treatments, offering significant commercial prospects. Tralokinumab, the first monoclonal antibody specifically targeting IL-13, has also been approved for moderate-to-severe AD.
Additionally, approved cytokine inhibitors, including Secukinumab (IL-17A) and Ustekinumab (IL-12/IL-23), although not primary AD treatments, have exhibited strong efficacy in psoriasis and other immune-mediated conditions, providing valuable reference points for potential AD applications.
Approved and Phase III Biologics for AD, Source: Pharmacodia
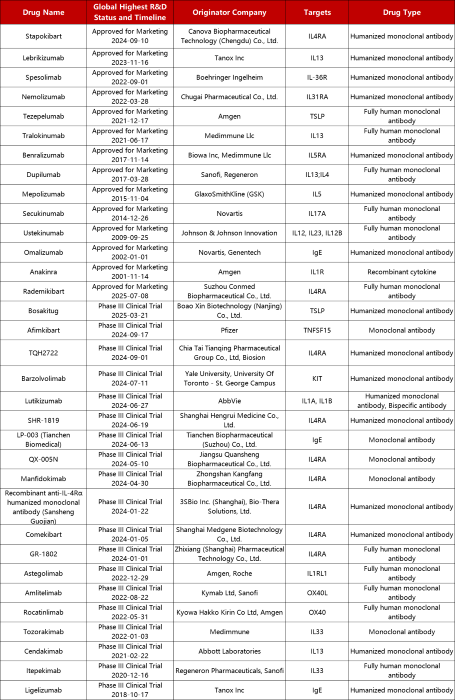
At the Phase III stage, numerous promising therapies continue to progress toward the market, with competition focusing on high-value targets such as IL-4Rα, TSLP, and OX40/OX40L. Stapokibart (Consino Biologics) became the first therapy approved in China in September 2024, backed by robust international Phase III data, positioning it as a potential competitor to Dupilumab.
Another IL-4Rα-targeting candidate, Rademikibart (Connect Biopharma), has already filed for regulatory approval. On the OX40 pathway, Rocatinlimab (Amgen, anti-OX40) and Amlitelimab (Sanofi, anti-OX40L) have both shown robust disease control in Phase II trials, increasing expectations for the next major advancement in AD treatment.
ACROBiosystems advances AD therapeutics with cutting-edge solutions
Building on these advances, ACROBiosystems has designed an extensive product portfolio for AD study, including highly active recombinant proteins, stable cell lines, and inhibitor screening kits.
These solutions encompass the full continuum of therapeutic advancement, including target identification and validation, candidate drug screening and development, and CMC production and quality control, accelerating the efficient translation of novel AD treatments from foundational research to clinical application.

Image Credit: ACROBiosystems
Hot AD target recommendations
Source: ACROBiosystems
| |
|
|
|
|
| IL-13 |
IL-4 R alpha |
TSLP |
OX40 Ligand |
IL-33 |
| OX40 |
IL-31 |
IL-18 |
IL-25 |
IL-4 |
| IgE Fc |
CD200 R1 |
IL-22 R alpha 1 |
IL-2 R alpha |
IL-17A |
| IL-1 alpha |
IL-1RL1 |
IL-2 |
IL-31 RA |
IL-5 |
| BTLA |
Fc epsilon RI alpha |
Fc gamma RIIB |
GITR |
IL-17F |
| IL-12/IL-23 p40 |
IL-12B |
IL-13 R alpha 1 |
IL-17 RB |
IL-1 beta |
| IL-22R |
IL-5 R alpha |
Siglec-8 |
... |
|
References and further reading:
- Ashutosh Pareek, et al. (2024). Unraveling Atopic Dermatitis: Insights into Pathophysiology, Therapeutic Advances, and Future Perspectives. Cells, 13(5), pp.425–425. https://doi.org/10.3390/cells13050425.
- Schuler, C.F., et al. (2024). Genetic and Immunological Pathogenesis of Atopic Dermatitis. Journal of Investigative Dermatology, 144(5), pp.954–968. https://doi.org/10.1016/j.jid.2023.10.019.
- Bieber, T., et al. (2022). Atopic dermatitis: pathomechanisms and lessons learned from novel systemic therapeutic options. Journal of the European Academy of Dermatology and Venereology, 36(9), pp.1432–1449. https://doi.org/10.1111/jdv.18225.
- Tsuge, M., et al. (2021). Current Insights into Atopic March. Children, 8(11), p.1067. https://doi.org/10.3390/children8111067.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.