
Image Credit: ACROBiosystems
1. Prevalence and risks of mycoplasma contamination
Mycoplasma contamination is a persistent issue in cell culture and biopharmaceutical manufacturing. Mycoplasmas are widespread in the environment and are commonly found on human mucosal surfaces.
These wall-less microorganisms are small, pleomorphic, and pathogenic, with over 190 species identified across humans, animals, and plants. Studies show that 15 to 35% of cell cultures may be contaminated, with rates reaching as high as 85% in certain laboratories. Major contamination sources include the environment and laboratory personnel.
2. Importance of mycoplasma testing in cell therapy
Mycoplasma testing is a crucial component of quality control in cell therapy studies. In clinical applications, such as those involving mesenchymal stromal cells (MSCs), several safety assessments are essential to guarantee consistent therapeutic outcomes, including mycoplasma detection.
Similarly, within CAR-T cell studies, mycoplasma contamination is recognized as a significant threat that compromises cell function and treatment safety. Contaminated cells reduce drug performance and may also present risks to patient health. Consequently, rigorous mycoplasma assessment is an indispensable safeguard for preserving the quality and safety of all clinical-grade cell products.
3. Impact of mycoplasma contamination
Mycoplasma contamination poses serious risks to cell cultures and biopharmaceutical manufacturing. It can lead to chromosomal abnormalities, interfere with nucleic acid synthesis and metabolism, and even induce apoptosis.
In manufacturing environments, mycoplasma contamination reduces yields, degrades quality, and, in severe cases, can necessitate entire batch disposal. The resulting downtime, equipment cleaning, and prolonged investigations are costly. Therefore, a comprehensive assessment of raw materials, culture processes, and final products is essential throughout biopharmaceutical development.
4. Limitations of conventional mycoplasma detection techniques
With the rapid advancement of cell therapies, effective mycoplasma analysis has become an essential stage in quality management. Conventional techniques, including culture assays, indicator cell assays, and polymerase chain reaction (PCR) evaluation, present notable limitations.
Culture-based assays may require up to 28 days to yield results, and they can display variable sensitivity, while indicator assays also exhibit inherent constraints. For the biopharmaceutical sector, where accelerated product release is crucial, such conventional methods are often insufficient.
5. Advantages of rapid detection technologies
Rapid nucleic acid amplification technologies, including quantitative real-time PCR (qPCR), have emerged as powerful alternatives to conventional mycoplasma analysis. qPCR enables precise identification within a shorter timeframe while providing elevated sensitivity, specificity, and affordability, making it particularly suitable for laboratory workflows.
However, qPCR cannot differentiate between live and dead mycoplasma, requires optimized experimental conditions, and must be accompanied by appropriate controls to reduce the likelihood of false positives or negatives.
To address these challenges and meet industry demands for reliable and rapid assessment, ACROBiosystems provides the Mycoplasma DNA Sample Preparation Kit (Cat. No. OPA-E101) and Mycoplasma Rapid Detection Kit (Cat. No. OPA-S102).
These kits comply with pharmacopeial standards in Europe and the United States. Their primer-probe designs encompass over 250 species of mycoplasma and related organisms. Featuring exceptional sensitivity and robust validation, the kits offer strong scientific support for the rapid release of biopharmaceutical products.

Image Credit: ACROBiosystems
The Mycoplasma Rapid Detection Kit (qPCR) demonstrates performance consistent with traditional culture-based and indicator cell-based approaches and has been verified by a third-party assessment conducted by Eurofins.
Validation data
- High sensitivity: Meets regulatory standards (10 CFU/mL).
Source: ACROBiosystems
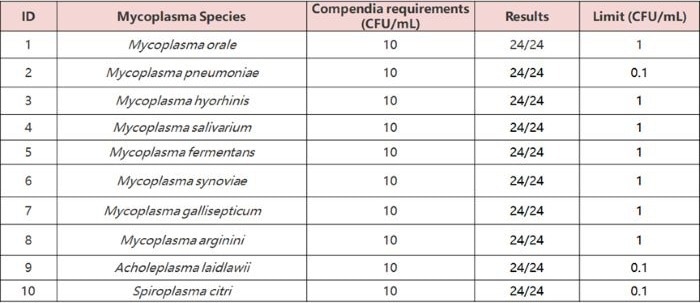
Tested 10 mycoplasma standards (10 CFU/mL) in 24 replicates each; all returned positive - meeting/exceeding EP 2.6.7 standards (10 CFU/mL).
- Broad coverage: Detects over 250 species of mycoplasma and mollicutes
Source: ACROBiosystems
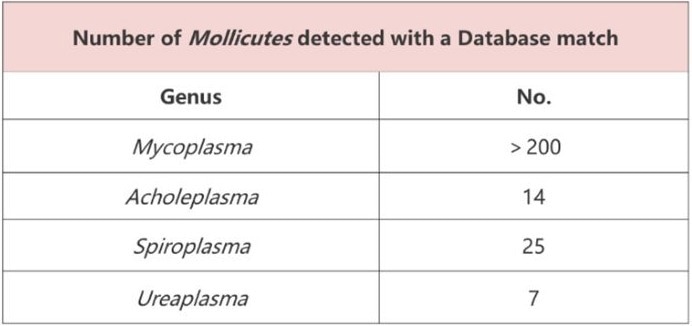

Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.