T-cell engager (TCE) treatment is a significant advancement in biomedicine, with transformative promise for treating autoimmune disorders and cancer.
TCEs function as "immune navigation systems" in autoimmune illnesses, specifically controlling hyperactive T cells and offering novel therapeutic options for conditions such as rheumatoid arthritis and type 1 diabetes.
In oncology, TCEs use bispecific or multispecific binding—targeting tumor-associated antigens and CD3 on T cells—to direct immune responses to tumor locations, resulting in substantial advances in treating hematologic malignancies and solid tumors.
Challenges in TCE development: Balancing efficacy and safety
Despite their potential for cancer treatment, TCEs confront significant challenges:
- On-target, off-tumor toxicity: Tumor-specific antigens are the optimal TCE targets. However, if the antigen is also produced at low levels in normal tissues, TCEs may cause T cells to attack healthy cells, resulting in serious consequences.
- Cytokine release syndrome (CRS): Rapid and excessive activation of T cells by TCEs can cause a cytokine storm, including IL-6, IFN-γ, and TNF-α, resulting in life-threatening symptoms such high temperature and hypotension.
- Narrow therapeutic window: TCEs have a restricted therapeutic window due to the need to balance antitumor effectiveness with toxicity.
As a result, precisely forecasting TCE efficacy, pharmacokinetics, and toxicity profiles in preclinical models—particularly CRS risk—directly impacts candidate medication success. Establishing animal models that accurately mimic human immune responses has become an essential necessity for TCE development.
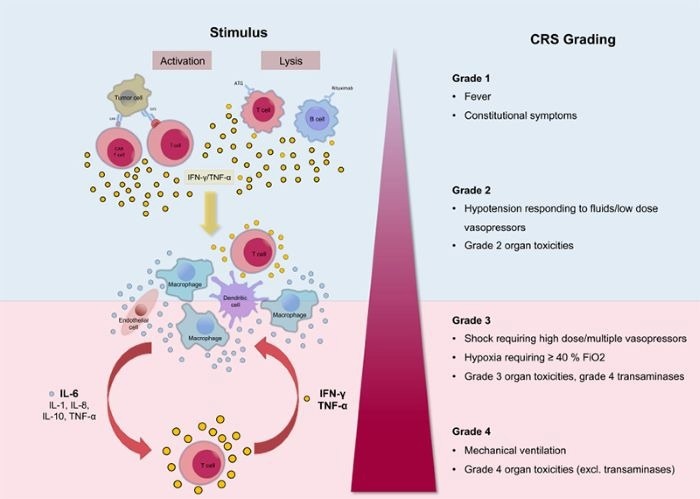
Mechanism of CRS. Image Credit: https://doi.org/10.1186/s40425-018-0343-9
The critical role of human-monkey cross-reactivity in TCE development
Animal studies are critical for proving TCE's efficacy and safety. Cynomolgus monkeys are a phylogenetically near model of humans, with high similarities in the amino acid sequence, spatial structure, and biological function of their CD3 protein. This makes them an important consideration for selecting relevant experimental models.
Chugai Pharmaceutical's recent research in the Journal of Applied Toxicology highlights the monkey model's particular usefulness in TCE safety assessments.
The study found that ERY22 (GPC3/CD3 TCE), a drug targeting GPC3-positive solid tumors, had a distinct dose-response profile in cynomolgus monkeys.
A single intravenous dose of 1000 μg/kg caused fatal CRS, while a stepwise dose-escalation regimen (1→3→10→30→100→300→1000 μg/kg) effectively prevented CRS while preserving partial tumor-killing activity.
This study demonstrates the monkey model's unique benefits in validating human-monkey cross-reactivity and provides a scientific foundation for optimizing TCE dosing techniques, easing the safety design of anti-tumor TCE medications.
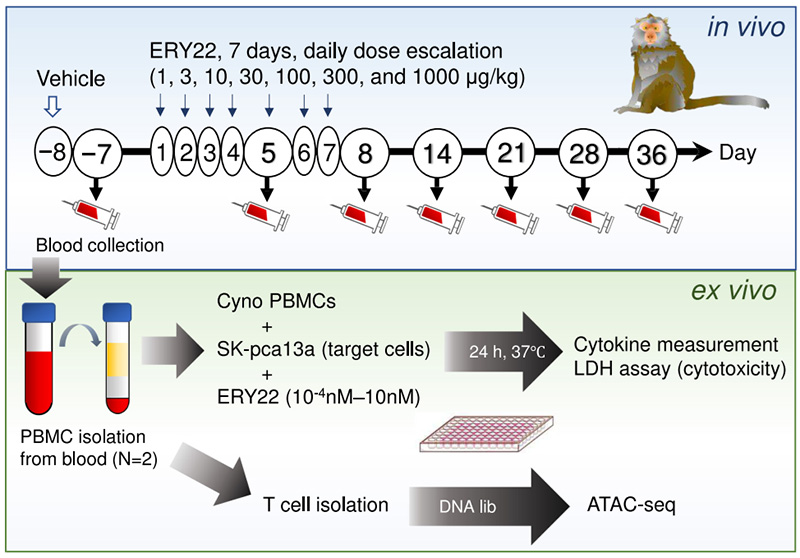
Experimental Scheme of Escalating ERY22 Dosing in Cynomolgus Monkey Model. Image Credit: https://doi.org/10.1002/jat.4812
High-quality cynomolgus CD3 protein facilitates innovative TCE drug development
High-quality cynomolgus CD3 protein allows for precise assessment of TCE cross-reactivity between humans and monkeys, allowing for the selection of relevant animal models and accelerating non-clinical evaluation.
ACROBiosystems provides high-quality cynomolgus monkey CD3 proteins, such as CD3E & CD3D, CD3E & CD3G heterodimers, and CD3 epsilon and CD3 delta monomers.
Using non-reducing electrophoresis and MALS, the CD3E&CD3D and CD3E&CD3G heterodimers were confirmed to be genuine 1:1 heterodimers, confirming protein purity and homogeneity.
ACROBiosystems’ CD3 proteins have been validated for binding activity with several TCEs (e.g., CD3×BCMA, CD3×CD20, CD3×DLL3) and clinical bispecific antibodies. This supports TCE drug screening and optimization, accelerating the discovery of new medicines.
Molecule list
Source: ACROBiosystems
| |
|
|
|
|
| CD3E & CD3D |
CD3E & CD3G |
CD3 epsilon |
CD3 delta |
CD3 gamma |

Image Credit: ACROBiosystems
Reference
- Shimabukuro-Vornhagen, A., et al. (2018). Cytokine release syndrome. Journal for ImmunoTherapy of Cancer, (online) 6(1). https://doi.org/10.1186/s40425-018-0343-9.
- Iwata, Y., et al. (2025). Antitumor Cytotoxic Activity Retained in Tolerized T Cells Following Daily Dose Escalation of a T‐Cell Engager in Cynomolgus Monkeys to Mitigate Cytokine Release Syndrome. Journal of Applied Toxicology, 45(9), pp.1844–1854. https://doi.org/10.1002/jat.4812.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.